Class 10 Science Chapter 2 Acids and Bases NCERT Solutions
Before getting into the details of NCERT Solutions For Class 10 Science Chapter 2 Acids And Bases, let’s have an overview of topics & subtopics under NCERT Solutions for Class 10 Science Chapter 2 Activities:
- Acids, Bases And Salts
- Understanding The Chemical Properties Of Acids And Bases
- What Do All Acids And All Bases Have In Common?
- How Strong Are Acid Or Base Solutions?
- More About Salts
Free download NCERT Solutions for Class 10 Science Chapter 2 Acids And Bases PDF in Hindi Medium as well as in English Medium for CBSE, Uttarakhand, Bihar, MP Board, Gujarat Board, and UP Board students, who are using NCERT Books based on updated CBSE Syllabus for the session 2019-20.
- NCERT Solutions for Class 10 Science Chapter 2 Intext Questions
- Acids, Bases and Salts Class 10 Page No 18
- Acids, Bases and Salts Class 10 Page No 22
- Acids, Bases and Salts Class 10 Page No 25
- Acids, Bases and Salts Class 10 Page No 28
- Acids, Bases and Salts Class 10 Page No 33
- Class 10 Chapter 2 Acids, Bases and Salts Chapter End Questions
- Class 10 Chapter 2 Acids, Bases and Salts MCQ (Multiple Choice Questions)
- अम्ल, क्षार एवं लवण कक्षा 10 विज्ञान हिंदी में
- Class 10 Acids Bases and Salts Important Questions
- Acids Bases and Salts Class 10 Notes
- Acids Bases and Salts NCERT Exemplar Solutions
- Acids, Bases and Salts Class 10 Extra Questions
NCERT Solutions for Class 10 Science Chapter 2 Intext Questions
Question 1
You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube ?
Answer:
(i) Put the red litmus paper in all the test tubes, turn by turn. The solution which turns red litmus to blue will be a basic solution. The blue litmus paper formed here can now be used to test the acidic solution.
(ii) Put the blue litmus paper obtained above in the remaining two test-tubes, turn-by-turn. The solution which turns the blue litmus paper to red will be the acidic solution.
(iii) The solution which has no effect on any litmus paper will be neutral and hence it will be distilled water.
Question 1
Why should curd and sour substances not be kept in brass and copper vessels ?
Answer:
Curd and sour substances should not be kept in brass and copper vessels because these and other sour food-stuffs contain acids which can react with the metal of the vessel to form poisonous metal compounds which can cause food poisoning and affect our health adversely.
Question 2
Which gas is usually liberated when an acid reacts with a metal ? Illustrate with an example. How will you test for the presence of this gas ?
Answer:
(i) Hydrogen (H2) gas is liberated when an acid reacts with a metal.
(ii) Illustration : Set up the apparatus as shown in the given figure. Take some zinc granules in the test tube. Add about 5 mL dilute hydrochloric acid slowly. Soon the reaction between zinc and hydrochloric acid starts and hydrogen gas is evolved.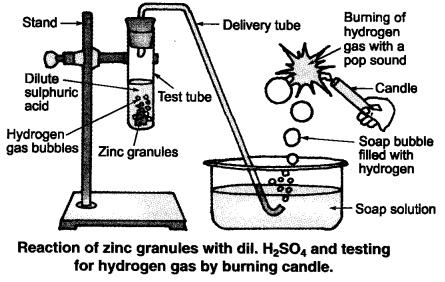
(iii) Test for H2 gas :
H2 gas is not soluble in water. When passed through soap solution, it gets trapped into bubbles.
Bring a burning candle near the soap bubble filled with gas. The soap bubble bursts and hydrogen gas burns with a pop sound.
Question 3
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Answer:
As the end product is calcium chloride and the gas formed is carbon dioxide, the metal compound A must be calcium carbonate. Therefore, the reaction between calcium carbonate and hydrochloric acid is![]()
Question 1
Why do HCl, HNO3, etc show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character ?
Answer:
H+ ions in aqueous solution are responsible for acidic character. HCl, HNO3, etc. give H+ ions in water while alcohol and glucose do not give H+ ion in water. Therefore, alcohol and glucose do not show acidic character.
Question 2
Why does an aqueous solution of an acid conduct electricity ?
Answer:
The aqueous solution of an acid conducts electricity due to the presence of charged particles called ions in it.
Question 3
Why does dry HCl gas not change the colour of the dry litmus paper ?
Answer:
Dry HCl gas does not give H+ ions and therefore does not change the colour of dry litmus paper.
Question 4
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid ?
Answer:
While diluting an acid it is recommended that the acid should be added to water and not water to the acid because if water is added to concentrated acid to dilute it, then a large amount of heat is evolved at once. This heat changes some of the water to steam explosively which can splash the acid on one’s face or clothes and cause acid burns.
Question 5
How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted ?
Answer:
When a given amount of an acid is added to water, there is a fixed number of hydronium ions per volume of the solution. On dilution, the number of hydronium ions per volume decreases and concentration decreases.
Question 6
How is the concentration of hydroxide ions (OH–) affected when excess base is dissolved in a solution of sodium hydroxide ?
Answer:
The concentration of hydroxide ions will increase when excess base is dissolved in a solution of sodium hydroxide, but it happens to a limited extent only after which the concentration becomes almost constant.
Question 1
You have two solutions A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of this is acidic and which one is basic ?
Answer:
A pH value of less than 7 indicates an acidic solution, while greater than 7 indicates a basic solution. Since solution A has more hydrogen ion concentration, solution A is acidic and solution B is basic.
Question 2
What effect does the concentration of H+ (aq) ions have on the nature of the solution ?
Answer:
More the concentration of H+ ions, higher the acidic nature of the solution.
Question 3
Do basic solutions also have H+ (aq) ions ? If yes, then why are these basic ?
Answer:
Basic solutions have H+ (aq) ions. But these are far less in number than OH– ions that is responsible for their basic nature.
Question 4
Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate) ?
Answer:
If the soil is too acidic (having low pH) then it is treated with materials like quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate).
Question 1
What is the common name of the compound CaOCl2 ?
Answer:
Bleaching powder.
Question 2
Name the substance which on treatment with chlorine yields bleaching powder.
Answer:
Slaked lime Ca (OH)2.
Question 3
Name the sodium compound which is used for softening hard water.
Answer:
Sodium carbonate.
Question 4
What will happen if a solution of sodium hydrogen carbonate is heated. Give the equation of the reaction involved ?
Answer:
Solution of sodium hydrogen carbonate on heating gives sodium carbonate and carbon dioxide gas is evolved.![]()
Question 5
Write an equation to show the reaction between plaster of Paris and water.
Answer: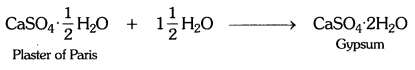
NCERT Solutions for Class 10 Science Chapter 2 Textbook Chapter End Questions
Question 1
A solution turns red litmus blue, its pH is likely to be
(a) 1
(b) 4
(c) 5
(d) 10
Answer:
(d) 10
Question 2
A solution reacts with crushed-egg shells to give a gas that turns lime water milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Answer:
(b) HCl
Question 3
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HC1. If we take 20 mL of the same solution of NaOH, the amount of HC1 solution (the same solution as before) required to neutralise it will be
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Answer:
(d) 16 mL
Question 4
Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
Answer:
(c) Antacid
Question 5
Write word equations and then balanced equations for the reaction taking place when
(a) dilute sulphuric acid reacts with zinc granules
(b) dilute hydrochloric acid reacts with magnesium ribbon
(c) dilute sulphuric acid reacts with aluminium powder
(d) dilute hydrochloric acid reacts with iron filing
Answer:
(a) Zinc + dilute sulphuric acid → Zinc sulphate + Hydrogen
Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
(b) Magnesium ribbon + dil. Hydrochloric acid → Magnesium chloride + Hydrogen
Mg (s) + 2 HCl (aq) → MgCl2 (aq) + H2 (g)
(c) Aluminium powder + dil. Sulphuric acid > Aluminium sulphate + Hydrogen
2Al (s) + 3H2SO4 (aq) → Al2 (SO4)3 (aq) + 3H2 (g)
(d) Iron filings + Dilute hydrochloric acid > Ferric chloride + Hydrogen
2Fe (s) + 6HCl (aq) → 2FeCl3 (aq) + 3H2 (g)
Question 6
Compounds such as alcohol and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
Answer:
Though compounds like alcohol and glucose contain hydrogen but they do not ionise in the solution to produce H+ ions on passing current through them.
(i) Take solutions of alcohols and glucose.
(ii) Fix two nails on a cork, and place the cork in 100 mL beaker.
(iii) Connect the nails to the two terminals of a 6 volt battery through a bulb and a switch, as shown in the given Figure.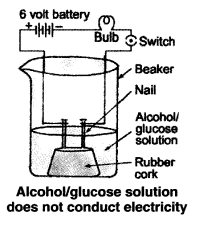
(iv) Now pour alcohol in the beaker and switch on the current.
(v) The bulb does not glow.
(vi) Repeat the experiment with glucose. The bulb does not glow in this case also.
(vii) This means no ions or H+ ions are present in the solution.
This shows that alcohols and glucose are not acids.
Question 7
Why does distilled water not conduct electricity, whereas rainwater does ?
Answer:
Distilled water does not conduct electricity because it does not contain any ionic compound (like acids, bases or salts) dissolved in it.
Rainwater, while falling to the earth through the atmosphere, dissolves an acidic gas carbon dioxide from the air and forms carbonic acid (H2CO3). Carbonic acid provides hydrogen ions, H+ (aq) and carbonate ions, CO(aq)32to rainwater. Hence, due to the presence of carbonic acid which provides ions to rainwater, the rainwater conducts electricity.
Question 8
Why do acids not show acidic behaviour in the absence of water ?
Answer:
The acidic behaviour of acids is due to the presence of hydrogen ions, [H+ (aq) ions], in them. The acid produces hydrogen ions only in the presence of water. So in the absence of water, an acid will not form hydrogen ions and hence will not show its acidic behaviour.
Question 9
Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11, 7 and 9 respectively. Which solution is
(a) Neutral
(b) Strongly alkaline
(c) Strongly acidic
(d) Weakly acidic
(e) Weakly alkaline
Arrange the pH in increasing order of hydrogen ion concentration.
Answer:
(a) D
(b) C
(c) B
(d) A
(e) E
Increasing order of hydrogen ion concentration
11 < 9 < 7 < 4 < 1
i. e., C < E < D < A < B
Question 10
Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. In which test tube will the fizzing occur more vigorously and why ?
Answer:
Fizzing will occur more vigorously in test tube A. Hydrochloric acid (HCl) is a strong acid whereas acetic acid (CH3COOH) is a weak acid. Being strong acid, the hydrochloric acid solution contains a much greater amount of hydrogen ions in it due to which the fizzing will occur more vigorously in test tube A (containing hydrochloric acid). The fizzing is due to the evolution of hydrogen gas which is formed by the action of acid on the magnesium metal of magnesium ribbon.
Question 11
fresh milk has a pH of 6. How do you think the pH will change as it turns into curd ? Explain your answer.
Answer:
pH of milk falls below 6 as it turns into curd due to the formation of lactic acid during this process. Lactic acid present in it reduces its pH value.
Question 12
A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline ?
(b) Why does this milk take a long time to set as curd ?
Answer:
(a) Milk is made slightly alkaline so that it may not get sour easily due to the formation of lactic acid in it.
(b) The alkaline milk takes a longer time to set into curd because the lactic acid being formed has to first neutralise the alkali present in it.
Question 13
Plaster of Paris should be stored in a moisture proof container. Explain why?
Answer:
Plaster of Paris should be stored in a moisture proof container because the presence of moisture can cause slow setting of plaster of Paris by bringing about its hydration. This will make the plaster of Paris useless after sometime.
Question 14
What is a neutralisation reaction ? Give two examples.
Answer:
The reaction between an acid and a base to form salt and water is called a neutralisation reaction.
Examples: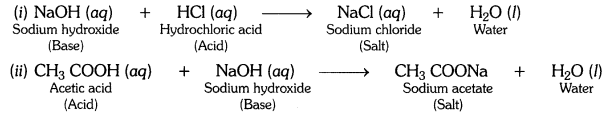
Question 15
Give two important uses of washing soda and baking soda.
Answer:
Uses of washing soda :
(i) Washing soda is used in glass, soap and paper industries.
(ii) It is used for removing permanent hardness of water.
Uses of baking soda :
(i) Baking soda is used as an antacid in medicines to remove acidity of the stomach.
(ii) Baking soda is used for making baking powder (used in making cakes, bread, etc.).
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 10 |
| Subject | Science |
| Chapter | Chapter 2 |
| Chapter Name | Acids, Bases, and Salts |
| Number of Questions Solved | 34 |
| Category | NCERT Solutions |
NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases, and Salts
NCERT Solutions for Class 10 Science (Chemistry) Chapter 2 Acids, bases, and salts are part of NCERT Solutions for Class 10 Science. Here we have given Class 10 Science NCERT Solutions Chapter 2.
Question 1
You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Solution:
The contents of each test tube would be identified by change in colour of red litmus paper. For example, when we wet the red litmus paper with the basic solution, it changes into blue colour. Put the changed blue litmus paper in the solution which turns the blue to red will be the acidic solution. The solution, which has no effect on any litmus paper, will be neutral and hence it will be distilled water.
Download NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts PDF
Question 2
Why should curd and sour substances not be kept in brass and copper vessels?
Solution:
Curd and other sour foodstuffs contain acids, which can react with the metal of the vessel to form poisonous metal compounds which can cause food poisoning and damage our health.
Question 3
Which gas is usually liberated when an acid reacts with a metal?
Solution:
When an acid reacts with metal, a salt and hydrogen gas is formed. i.e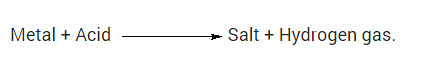
Question 4
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Solution:
The gas that extinguishes a burning candle is carbon dioxide, which is formed by the action of dilute hydrochloric acid on a metal carbonate and produces effervescence. Now, since one of the compounds formed is calcium chloride, it shows that the metal compound is calcium carbonate. Thus, the metal compound A is calcium carbonate (CaCO3). Calcium carbonate reacts with dilute hydrochloric acid to form calcium chloride, carbon dioxide and water. This can be written as:
Question 5
Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Solution:
An acid is a substance, which dissociates on dissolving in water to produce hydrogen ions [H+(aq) ions]. The acids like HCl, H2SO4, HNO3 and CH3COOH, etc., show acidic character because they dissociate in aqueous solutions to produce hydrogen ions, H+(aq) ions.
The compounds such as glucose and alcohol also contain hydrogen but they do not show acidic character. The aqueous solutions of glucose and alcohol do not show acidic character because the hydrogen in them does not separate out as hydrogen ions [H+ (aq) ions] on dissolving in water.
Question 6
Why does an aqueous solution of acid conduct electricity?
Solution:
The aqueous solution of an acid conducts electricity due to the presence of charged particles called ions in it.
Question 7
Why does dry HCl gas not change the colour of the dry litmus paper?
Solution:
Dry HCl gas does not contain any hydrogen ions in it, so it does not show acidic behaviour. In fact, dry HCl gas does not change the colour of dry litmus paper as it has no hydrogen ions [H+(aq) ions] in it.
Question 8
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Solution:
Diluting an acid should be done by adding concentrated acid to water gradually with stirring and not by adding water to concentrated acid.
The heat is evolved gradually when a concentrated acid is added to water for diluting an acid and the large amount of water is easily absorbed.
If, however, water is added to concentrated acid to dilute it, a large amount of heat is evolved at once. The heat generated may cause the mixture to splash the acid on our face or clothes and cause acid burns.
Question 9
How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
Solution:
When the concentrated solution of an acid is diluted by mixing water, the concentration of hydronium ions H3O+ per unit volume decreases.
Question 10
How is the concentration of hydroxide ions (OH-) affected when excess base is dissolved in water?
Solution:
When the solution of a base is diluted by mixing more water in it, the concentration of hydroxide ions (OH– ions) per unit volume decreases.
Question 11
You have two solutions A and B. The pH of solution A is 6 and pH of solution B is 8.
i. Which solution has more hydrogen ion concentration?
ii. Which of this is acidic and which one is basic?
Solution:
The pH of a solution is inversely proportional to its hydrogen ion concentration. This means that the solution having lower pH will have more hydrogen ion concentration. In this case, solution A(having a lower pH of 6) will have more hydrogen ion concentration.Solution A is acidic and solution B is basic.
Question 12
What effect does the concentration of H+(aq) ions have on the nature of the solution?
Solution:
Acids produce hydrogen ions in water. So, when an acid is added to water, the concentration of hydrogen ions in water increases. The solution of acid thus formed will have more of hydrogen ions and it will be acidic in nature.
Question 13
Do basic solutions also have H+(aq) ions? If yes, why are these basic?
Solution:
No, the basic solution doesn’t have H+ ions as the solution has excess of hydroxide ions.
Question 14
Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Solution:
Most often the soil in the fields is too acidic. If the soil is too acidic (having low pH), it is treated with materials like quicklime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate). Thus, a farmer should add lime or slaked lime in his fields when the soil is too acidic.
Question 15
What is the common name of the compound CaOCl2?
Solution:
The common name of the compound CaOCl2 is bleaching powder.
Question 16
Name the substance that on treatment with chlorine yields bleaching powder.
Solution:
Calcium hydroxide is the substance that on treatment with chlorine yields bleaching powder.
Question 17
Name the sodium compound, which is used, for softening hard water.
Solution:
Sodium carbonate (washing soda) is used for softening hard water.
Question 18
What will happen if a solution of sodium hydro carbonate is heated? Give the equation of the reaction involved.
Sodium carbonate and carbon dioxide are evolved when sodium hydro carbonate is heated.![]()
Question 19
Write an equation to show the reaction between plaster of Paris and water.
Solution:
Plaster of Paris has a very remarkable property of setting into a hard mass on wetting with water. So, when water is added to plaster of Paris, it sets into a hard mass in about half an hour. The setting of plaster of Paris is due to the hydration crystals of gypsum, which set to form a hard, solid mass.
Question 20
Why does distilled water not conduct electricity, whereas rainwater does?
Solution:
Distilled water does not conduct electricity because it does not contain any ionic compound (like acids, bases or salts) dissolved in it. On the other hand, rain water conducts electricity. This can be explained as follows: Rain water, while falling to the earth through the atmosphere, dissolves an acidic gas carbon dioxide from the air and forms carbonic acid (H2CO3). Carbonic acid provides hydrogen ions, H+(aq), and carbonate ions, CO2-3 (aq), to rain water. So, due to the presence of carbonic acid (which provides ions to rain water), the rain water conducts electricity.
Question 21
Why do acids not show acidic behaviour in the absence of water?
Solution:
The acidic behaviour of acid is due to the presence of hydrogen ions. The acids will not show its acidic behaviour in the absence of water, this is because the acids produce hydrogen ions only in the presence of water.
Question 22
Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11, 7 and 9 respectively. Which solution is?
(i) Neutral?
(ii) Strongly alkaline?
(iii) Strongly acidic?
(iv) Weakly acidic?
(v) Weakly alkaline? Arrange the pH in increasing order of hydrogen-ion concentration.
Solution: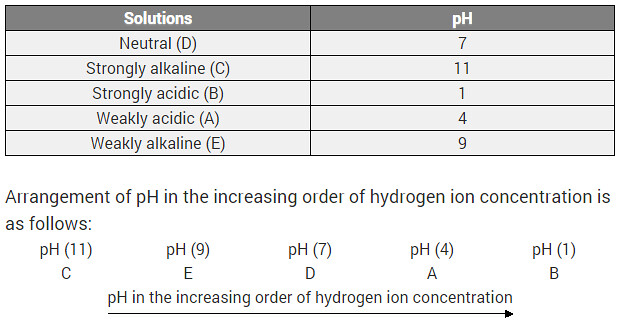
Question 23
Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test-tube A while acetic acid (CH3COOH) is added to test-tube B. In which test-tube will fizzing occur more vigorously and why?
Solution:
Acetic acid (CH3COOH) is a weak acid whereas hydrochloric acid (HCl) is a strong acid. Fizzing occurs in the test tube due to the evolution of hydrogen gas by the action of acid on magnesium ribbon. Since hydrochloric acid is a strong acid a large amount of hydrogen gas is liberated in the test tube A. So fizzing occurs more vigorously in test tube A .
Question 24
Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain.
Solution:
The pH will change to below 6, as lactic acid is formed when milk turns into curd.
Question 25
Plaster of Paris should be stored in a moisture-proof container. Why?
Solution:
The presence of moisture can affect the slow setting of plaster of Paris by bringing about its hydration. This will make the plaster of Paris useless after some time.
Question 26
What is a neutralization reaction? Give two examples.
Solution:
The reaction of an acid and a base, giving rise to the corresponding salt and water is called neutralization reaction.
Examples:
NaOH + HCl → NaCl + H2O
Mg(OH)2 + H2CO3 → MgCO3 + 2H2O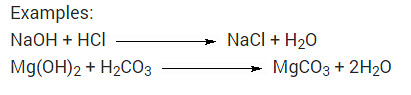
Question 27
Give two important uses of washing soda and baking soda.
Solution:
Washing soda
1. It is often used as an electrolyte.
2. Domestically it is used as a water softener during laundry.
Baking soda
1. It is used to test garden soil for acidity. If it develops bubbles, the soil is too acidic.
2. Washing a car with it can remove dead bug bodies without damaging the paint.
NCERT Solutions for Class 10 Science Chapter 2 (MCQs) [1 Mark each]
Question 1.
Equal pieces of zinc granules are dropped in four test tubes. Following substances are poured in all the four test tubes. The reaction will be vigorous with [CCE 2014]
(a) CH3COOH
(b) HCl
(c) sodium bicarbonate solution
(d) lemon juice
Answer:
(b) Strong adds like HCl react vigorously with active metals like Zn and form metal salt and evolve H2 gas.![]()
Question 2.
Which of the following statements shows the property of an acid? [CCE 2014]
(a) It turns blue litmus to red
(b) It is sour in taste
(c) It has no effect on red litmus
(d) All of the above
Answer:
(d) An acid turns blue litmus red. Thus, it has no effect on red litmus and acids are sour in taste.
Question 3.
A drop of a liquid sample was put on the pH paper. It was observed that the colour of the pH paper turned blue. The liquid sample is [CCE 2014]
(a) lemon juice
(b) sodium bicarbonate solution
(c) distilled water
(d) hydrochloric acid
Answer:
(b) The liquid sample is of sodium bicarbonate (NaHC3) solution. It is a basic solution. And we know that a basic solution turns pH paper blue.
Question 4.
Two solutions X and Y were found to have pH value of 4 and 10 respectively. The inference that can be drawn is [CCE 2014]
(a) X is a base and Y is an acid
(b) Both X and Y are acidic solutions
(c) X is an acid and Yis a base
(d) Both X and Y are bases
Answer:
(c) Any solution having pH > 7 will be a base while the solution having pH < 7 will surely be an acid. Hence, it can be concluded that X is an acid (pH=4, i.e. < 7) and Yis a base (pH =10, i.e. > 7).
Question 5.
A student was asked to collect apparatus from lab store, for doing experiment of pH of given sample. Identify the article which he is not supposed to pick. [CCE 2014]
(a) pH paper
(b) Dropper
(c) Litmus paper
(d) Petri dish
Answer:
(d) Petri dish is not required for doing experiment of pH.
Question 6.
Which one of the following would you need to identify the gas that evolve when you heat NaOH solution with zinc metal? [CCE 2014]
(a) Red litmus solution
(b) Blue litmus solution
(c) A burning splinter / matchstick
(d) Lime water
Answer:
(c) When a base like NaOH is treated with any active metal like Zn, it produces H2 gas. And the presence of the hydrogen gas can be tested by bringing a burning splinter/ matchstick near the gas produced. The gas will burn with a pop sound confirming the presence of hydrogen gas.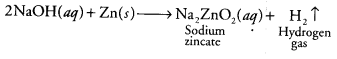
Question 7.
A solution has pH value of 5. On adding 10 mL of NaCl to it, what will be the pH of the new solution?
(a) More than 5
(b) Less than 5
(c) Only seven
(d) No change in pH
Answer:
(d) As NaCl is a neutral solution due to its complete ionization in H2O and has pH = 7. Thus, it will not affect the pH of the solution. Hence, the pH of the solution will remain unaffected.
Question 8.
A salt is dissolved in water. The pH of this salt solution was found to be 7 by measuring the pH with a universal indicator paper. The salt is most likely to be
(a) Na2CO3
(b) KCl
(c) NH4Cl
(d) CH3COONa
Answer:
(b) Salts which are completely ionisable in water are said to be neutral salts and their pH is equal to 7. Among the given salts, only KCl is completely ionisable in the following manner: KCl (aq) → K+ + Cl
Hence, the pH of KCl will be 7.
Question 9.
A student performed an experiment using zinc granules and sodium carbonate with sodium hydroxide and hydrochloric acid under different
conditions as shown below.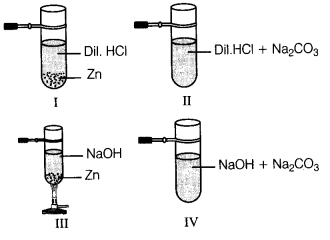
In which set up, no gas is evolved?
(a) I
(b) II
(c) III
(d) IV
Answer:
(d) Gas will not evolve in the case of IV set up as NaOH does not react with sodium carbonate.
Question 10.
Four set ups as given below are arranged to identify the gas evolved when dilute hydrochloric acid was added to zinc granules. Which is the most appropriate set up?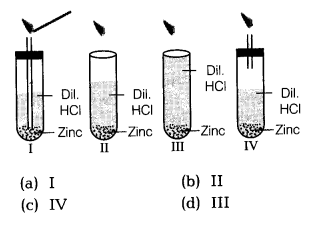
(a) I
(b) II
(c) IV
(d) III
Answer:
(c) The gas evolved can be tested as shown in IV set up because to evolve H2 gas delivery tube, should not dip in the acid.
Question 11.
Four students I, II, III and IV were asked to examine the changes for blue and red litmus paper strips with dil. HCl (Solution A) and dil. NaOH (Solutions). The following observations were reported by the 4 students. The sign …………. indicates no colour change. [CCE 2015 ]
| Litmus | A | B | Litmus | A | B | |
| I | Blue | — | red | Blue | red | — |
| II | Red | — | blue | Red | — | blue |
| III | Blue | red | red | Blue | blue | blue |
| IV | Red | blue | blue | Red | red | red |
The correct observation would be of student
(a) I
(b) II
(c) III
(d) IV
Answer:
(c) The correct observation is taken by student III because HCl (solution A) is an acid which turns blue litmus red and dil. NaOH (solution B) is a base which turns red litmus blue.
Question 12.
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish orange. Which of the following would change the colour of this pH paper to greenish blue? [NCERT Exemplar]
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer:
(d) As pH paper turns greenish blue for weakly basic compound and antacids contain weak base like Mg(OH)2. So, an antacid would change the colour of this pH paper to greenish blue. Other options (a) and (b) contain acids and option (c) is a neutral salt.
NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts (Hindi Medium)
























Class 10 Science Acid, Bases and Salts Mind Map
Acids
- Produce [H+] in H2O
- Sour taste
- Turns blue litmus red
- Acts as electrolyte in solution
Chemical Properties Of Acids
Acids + Metal → Salt + H2
e.g. H2SO4 + Zn → ZnSO4 + H2
Acids + Metal Carbonate/Metal hydrogen
Carbonate = Salt + CO2
e.g NaHCO3 + HCl → NaCl + H2O + CO2
Acids + Bases → Salt + water
NaOH + HCl → NaCl + H2O
Acids + Metal oxide → Salt + Water
H2SO4 + CuO → CuSO4 + H2O
Bases
- Produce [OH ] in H2O
- Bitter taste
- Turns red litmus blue
- Acts as electrolyte in solution
- Water soluble bases are known as alkali
Chemical Properties Of Bases
Bases + Metals → Salt + H2
2NaOH + Zn → Na2ZnO2 + H2
Bases + Acids → Salt + Water
KOH + HCl → KCl + H20
Base + Non-metallic oxide → Salt + water
2NaOH + CO2 → Na2CO3 + H2O
Salt
Salt is formed by combination of acid and base through neutralization reaction.
water of Crystallization :
It is the fixed number of water molecules present in one formula unit of a salt e.g.
CuSO4.5H2O, Na2CO3.10H2O
Types of Salts
The acidic & basic nature of salts depends on the acid and base combined in neutralization reaction.
| pH | Acid | Base | Salt | Example |
| 7 | Strong | Strong | Neutral | NaOH + H2SO4 → Na2SO4 + H2O |
| < 7 | Strong | Weak | Acidic | HCl + NH4OH → 4 NH4Cl + H2O |
| > 7 | Weak | Strong | Basic | CH3COOH + KOH → 4 CH3COOK + H2O |
| < 7 | Weak | Weak | Weak | CH3COOH + NH4OH → CH3COONH4 + H2O |
Some Important Salts
Plaster of Paris (CaSO4. 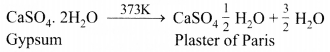
Used for making toys, material for decor action, smooth surfaces.
Bleaching Powder (CaOCl2):
Ca(OH)2 + Cl2 → CaOCl2 + H2O
dry slaked lime
Used
- as an oxidising agent in chemical industry.
- In disinfecting water.
Common Salt NaCl:
- Main source is sea water.
- Also exists in the form of rock hence also known as rock salt.
- Important component of food.
- Used in preparation of sodium hydroxide, baking soda & washing soda.
Baking Soda (NaHCO3)
- NaCl + H2O + CO2 + NH23 → NH4Cl + NaHCO3
- Mild non – corrosive
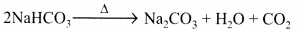
- Used as in baking cakes as antacid
- Fire extinguisher
Sodium Hydroxide (NaOH)
Prepared by chlor alkali process .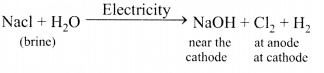
Washing Soda (Na2CO3. 10H2O) :
• Na2CO3 + 10H2O → 4 Na2CO3. 10H2O
• Used in glass, soap & paper indusby, removing Permanent hardness of water and cleaning agent
Indicators
| Indicators | Color in Acidic medium | Color in Basic Medium |
| Litmus solution | Red | Blue |
| Methyl orange | Pink | Orange |
| Phenolphthalein | Colourless | Pink |
| Methyl red | Yellow | Red |
These are the substances which indicate the acidic or basic nature of the solution by their colour change.
Strength of Acids and Bases in Solution
- Some animals like bee and plants like nettle secretes highly acidic substance for self defense.
- Lower pH of sour & sweet food can cause tooth decay. The pH of mouth should be more than 5.5
- The inner lining of stomach protects vital cells from the acidic pH which is developed by HCl secreted by stomach.
The optimum pH range for human body is 7 to 7.8
| πH = 7 | Neutral Solution | H3O+ = OH– |
| πH > 7 | Basic Solution | OH– > H3O+ |
| πH < 7 | Acidic Solution | OH– < H3O+ |
Addition of Acids or Bases to Water
Always add acid to water and not water to acid because this process is highly exothermic. The acid must be added slowly to water by constant shirring on. Adding water to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns.
Now that you are provided all the necessary information regarding NCERT Solutions for Class 10 Science Chapter 2 Activities and we hope this detailed article on NCERT Solutions For Class 10 Science Chapter 2 Acids And Bases is helpful. If you have any query regarding this article or Science Class 10 Chapter 2 Acids And Bases, drop your comments in the comment box below and we will get back to you as soon as possible.
Important Questions of Acids Bases and Salts Class 10 Science Chapter 2
Question 1.
With the help of an example explain what happens when a base reacts with a non- metallic oxide. What do you infer about the nature of non-metal oxide? (Board Term I, 2017)
Answer:
Oxides of non-metals react with bases to form salt and water. For example, the reaction between carbon dioxide and calcium hydroxide. Calcium hydroxide, which is a base, reacts with carbon dioxide to produce salt and water.
Hence, oxides of non-metals are acidic in nature.
Question 2.
What is observed when carbon dioxide gas is passed through lime water
(i) for a short duration?
(ii) for a long duration? Also write the chemical equations for the reactions involved. (Board Term I, 2016)
Answer:
(i) When CO2 is passed through lime water for short interval of time, it turns milky due to the formation of insoluble calcium carbonate.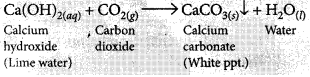
(ii) If CO2 is passed for long duration through lime water, the white precipitate formed dissolves due to the formation of soluble calcium hydrogen carbonate and the solution becomes clear.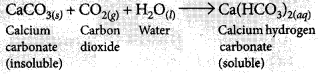
Question 3.
2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the content are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation of the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with dilute solution of a strong acid.
Answer:
It is observed that active metals like zinc react with strong bases like NaOH, KOH etc. to liberate hydrogen gas and corresponding salt.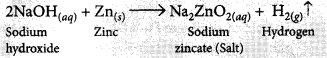
The evolution of gas is confirmed by the bubble formation in soap solution.
Test to detect H2 gas: When burning matchstick is kept on the mouth of this test tube, pop sound is heard which confirms the presence of H2 gas. When Zn metal reacts with dilute solution of strong acid, H2 gas is evolved.![]()
Question 4.
Write the names of the product formed when zinc reacts with NaOH. Also write the balanced chemical equation for the reaction involved. Write a test to confirm the presence of the gas evolved during this reaction. (Board Term I, 2015)
Answer:
Refer to answer 3.
Question 5.
To. a solution of sodium hydroxide in a test tube, two drops of phenolphthalein are added.
(i) State the colour change observed.
(ii) If dil HCl is added dropwise to the solution, what will be the colour change?
(iii) On adding few drops of NaOH solution to the above mixture the colour of the solution reappears. Why? (Board Term I, 2013)
Answer:
(i) On adding phenolphthalein to NaOH solution, the colour becomes pink.
(ii) On adding dilute HCl solution dropwise to the same test tube, the pink colour disappears and the solution again becomes colourless.
(iii) On again adding NaOH to the above mixture, pink colour reappears because the medium becomes basic again.
Question 6.
A cloth’strip dipped in onion juice is used for testing a liquid ‘X. The liquid ‘X changes its
odour. Which type of an indicator is onion juice? The liquid ‘X turns blue litmus red. List the observations the liquid ‘X will show on reacting with the following :
(a) Zinc granules
(b) Solid sodium carbonate
Write the chemical equations for the reactions involved.
Answer:
Onion juice is an olfactory indicator. Olfactory indicators give one type of odour in acidic medium and a different odour in basic medium. As the liquid ‘X’ turns blue litmus red, hence it is an acidic solution.
(a) Acids react with active metals such as zinc, magnesium etc. and evolve hydrogen gas, for example,
Zn(s) dil.H2SO4(aq) → ZnSO4H2(g)
(b) Acids react with metal carbonates to give carbon dioxide with brisk effervescence.
For example, Na2CO3 + H2SO4 → Na2SO4 + CO2 + H2O
Question 7.
(a) Write the chemical name and formula of marble.
(b) It has been found that marbles of Taj are getting corroded due to development of industrial areas around it. Explain this fact giving a chemical equation.
(c) (i) What happens when CO2 is passed through lime water?
(ii) What happens when CO2 is passed in excess through lime? (Board Term I, 2013)
Answer:
(a) The chemical formula of marble (lime stone) is CaCO3. Its chemical name is calcium carbonate.
(b) Taj Mahal, one of the seven wonders of the world situated at Agra, is continuously losing its luster day by day due to rapid industrialisation which causes acid rain.
The sulphuric acid present in the acid rain causes the marble (CaCO3) to be washed off as calcium sulphate (CaSO4), leading to the deterioration of such a splendid piece of architecture.
CaCO3(s) + H2SO4(aq) → CaSO4(aq) + H2Ol + CO2(g)
(c) Refer to answer 2.
Question 8.
On diluting an acid, it is advised to add acid to water and not water to acid. Explain why it is so advised? (Board Term I, 2014)
Draw a labelled diagram to show the preparation of hydrogen chloride gas in laboratory.
(ii) Test the gas evolved first with dry and then with wet litmus paper. In which of the two cases, does the litmus paper show change in colour?
(iii) State the reason of exhibiting acidic character by dry HCl gas/HCl solution.
Answer:
Diluting a concentrated acid with water is a highly exothermic process. So, when water is added to concentrated acid, large amounts of heat is liberated which changes some water to steam explosively which can splash the acid and even the glass apparatus may break due to excessive heating.
Question 9.
(i) Draw a labelled diagram to show the preparation of hydrogen chloride gas in laboratory.
(ii) Test the gas evolved first with dry and then with wet litmus paper. In which of the two cases, does the litmus paper show change in colour?
(iii) State the reason of exhibiting acidic character by dry HCl gas/HCl solution. (2020)
Answer: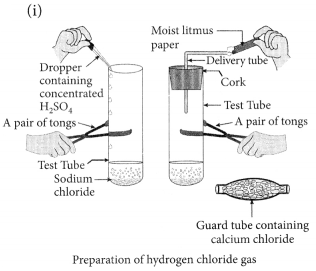
(ii) There is no change in the colour of ‘dry’ blue litmus paper but ‘moist’ blue litmus paper turns red if brought near the mouth of the test tube.
This shows that HCl gas does not show acidic behaviour in absence of water but it shows acidic behaviour in presence of water.
(iii) When HC1 gas dissolves in water, forms hydrochloric acid solution i.e., HCl(aq) which then produces H+(aq) or H3O+(aq) ions.
HCl + H2O → H3O+ + Cl–
Due to the presence of H+ or H3O+ it shows acidic behaviour.
Question 10.
Complete and balance the following chemical equations :
(i) NaOH(aq) + Zn(s) →
(ii) CaCO3(s) + H2O(l) + CO2(g) →
(iii) HCl(aq) + H2O(l) →
Answer:
(i) 2NaOH(aq) + Zn(s) → Na2ZnO2(aq) + H2(g)
(ii) CaCO3(s) + CO2(g) + H2Ol → Ca(HCO3)2(aq)
(iii) HCl(aq) + H2Ol > H3O+ Cl–(aq)
Question 11.
How the following substances will dissociate to produce ions in their solutions?
(i) Hydrochloric acid
(ii) Nitric acid
(iii) Sulphuric acid
(iv) Sodium hydroxide
(v) Potassium hydroxide
(vi) Magnesium hydroxide (Board Term 1, 2017)
Answer:
Dissociation of various substances to produce ions in their solutions are :
(i) Hydrochloric acid (HCl):
HCl(aq) ⇌ H+(aq) + Cl–(aq)
(ii) Nitric acid (HNO3
HNO3(aq) ⇌ + H+aq + NO–3(aq)
(iii) Sulphuric acid (H2SO4):
H2SO4(aq) ⇌ 2H+(aq) + SO2-4(aq)
(iv) Sodium hydroxide (NaOH):
NaOH(aq) ⇌ Na+(aq) + OH–(aq)
(v) Potassium hydroxide (KOH) :
KOH(aq)⇌ K+(aq) + OH–(aq)
(vi) Magnesium hydroxide [Mg(OH)2] :
Mg(OH)2(aq) ⇌ Mg2++(aq) + 2OH– (aq)
Question 12.
Sugandha prepares HCl gas in her school laboratory using certain chemicals. She puts both dry and wet blue litmus papers in contact with the gas.
(i) Name the reagents used by Sugandha to prepare HCl gas.
(ii) State the colour changes observed with the dry and wet blue litmus papers.
(iii) Show the formation of ions when HCl gas combines with water. (Board Term I, 2013)
Answer:
(i) Dense white fumes of hydrogen chloride gas are evolved on heating solid sodium chloride with concentrated sulphuric acid.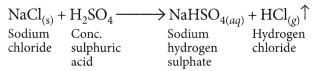
(ii) Refer to answer 9(ii).
(iii) Refer to answer 9(iii).
Question 13.
(a) Illustrate an activity to investigate whether all compounds containing hydrogen are acidic.
(b) What happens when hydrochloric acid and sodium hydroxide are dissolved in water. Explain by giving equation of each. (Board Term 1, 2016)
Answer:
(a) Take two beakers, one containing HCl acid and other containing alcohol which is not an acid but contains hydrogen. Now, fix two iron nails on a rubber cork and insert in a beaker and connect the nail to the two terminal of 6V battery through a switch and a bulb. Pour some dilute HCl solution in beaker and switch on the current. The bulb starts glowing. This shows that acids get dissociated as H+ and Cl– ions and these ions are responsible for conducting electricity.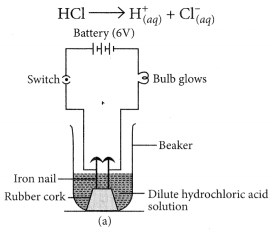
Let us now take alcohol solution in the beaker and switch on the current. The bulb does not glow in this case. This shows that alcohol does not conduct electricity.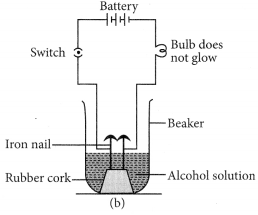
So, all acids have hydrogen but all hydrogen containing compounds are not acid.
(b) HCl dissociates in aqueous solution to give hydrogen ions (or hydronium ions) and chloride ions.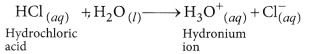
NaOH when dissolved in water produces sodium ions and hydroxide ions in the solution.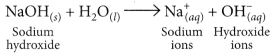
Question 14.
An aqueous solution ‘A’ turns phenolphthalein solution pink. On addition of an aqueous solution ‘B’ to ‘A’ the pink colour disappears. The following statement is true for solution ‘A’ and ‘B’:
(a) A is strongly basic and B is a weak base.
(b) A is strongly acidic and B is a weak acid.
(c) A has pH greater than 7 and B has pH less than 7.
(d) A has pH less than 7 and B has pH greater than 7. (2020)
Answer:
(c) As the aqueous solution of A turns phenolphthalein solution pink, hence A is basic in nature. On adding an acidic solution, the pink colour will disappear. Hence, B is an acid.
Question 15.
Out of HCl and CH3COOH, which one is a weak acid and why? Explain with the help of an example. (AI 2019)
Answer:
Out of HCl and CH3COOH, CH3COOH is a weak acid because it dissociates partially in the solution. This can be proved with the help of following example.
If 1 M HCl and 1 M CH3COOH are taken in the beaker as shown in the figure, greater deflection is observed in case of HCl which shows that more ions are produced by HCl in solution which produce more current.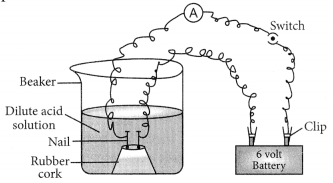
Question 16.
Explain how an antacid works. (Board Term I, 2017)
Answer:
The acidity produced due to excess hydrochloric acid in the stomach which cause indigestion, produce pain and irritation. Milk of magnesia (chemically magnesium hydroxide) is used as an antacid. Since, it is basic in nature, reacts with the excess hydrochloric acid present in the stomach and neutralises it.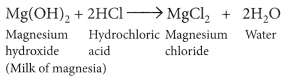
Question 17.
(a) Three acidic solutions A, B and C have pH = 0, 3 and 5 respectively.
(i) Which solution has highest concentration of H+ ions?
(ii) Which solution has the lowest concentration of H+ ions?
(b) How concentrated sulphuric acid can be diluted? Describe the process. (Board Term I, 2014)
Answer:
(a) (i) The solution having lower pH will have more hydrogen ion concentration. Hence, solution ‘A’ will have highest H+ ion concentration.
(ii) Solution CC’ i.e., pH = 5 has the lowest concentration of H+ ions.
(b) Mixing of an acid with water is called dilution. This process is highly exothermic and therefore, acid is always added to the water not water to acid. The process for diluting concentrated sulphuric acid is :
(i) Take about 10 mL of water in a beaker.
(ii) Add concentrated sulphuric acid dropwise to water and swirl the beaker slowly.
Question 18.
A compound P forms the enamel of teeth. It is the hardest substance of the body. It doesn’t dissolve in water but gets corroded when the pH is lowered below 5.5.
(a) Identify the compound P.
(b) How does it undergo damage due to eating chocolate and sweets? What should we do to prevent tooth decay? (Board Term I, 2014, 2013)
Answer:
(a) The compound P is calcium phosphate, (b) Eating chocolates and sweets produce large amount of acid in the mouth which is not completely neutralised by the saliva produced in the mouth. Excess acid attacks the enamel and tooth decay starts as pH of the mouth falls below 5.5. The best way to prevent tooth decay is to clean the teeth by using toothpastes after eating food. Toothpastes which are generally basic neutralise the excess acid in the mouth.
Question 19.
Baking soda is a mixture of
(a) sodium carbonate and acetic acid
(b) sodium carbonate and tartaric acid
(c) sodium hydrogen carbonate and tartaric acid
(d) sodium hydrogen carbonate and acetic acid.
Answer:
(c) : Baking soda is a mixture of sodium hydrogen carbonate and a mild edible acid like tartaric acid or citric acid.
Question 20.
The chemical formula for plaster of Paris is
(a) CaSO4.2H2O
(b) CaSO4.2H2O
(c) CaSO4.
(d) 2CaSO4.2H2O
Answer:
(c, d) : Plaster of Paris is calcium sulphate hemihydrate which can be represented as,
CaSO4.
Question 21.
“Sodium hydrogen carbonate is a basic salt”. Justify this statement. How is it converted into washing soda? (AI2019)
Answer:
Sodium hydrogen carbonate (NaHCO3) is basic in nature as on hydrolysis it gives a mixture of strong base (NaOH) and weak acid (H2CO3). Sodium hydrogen carbonate is converted to washing soda in the following way:
(i) Thermal decomposition of NaHCO3: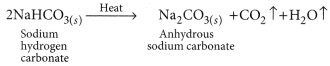
(ii) Recrystallisation of sodium carbonate:![]()
Question 22.
Write the chemical formula of Bleaching powder. How is bleaching powder prepared? For what purpose is it used in drinking water? (Board Term I, 2016)
Answer:
The chemical formula of bleaching powder is CaOCl2.
It is prepared by the action of chlorine gas on dry slaked lime Ca(OH)2.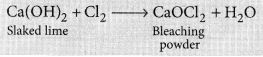
The chlorine used in the above reaction is the by-product produced during the electrolysis of brine. It is used in disinfecting drinking water as chlorine liberated by it, kills the germs.
Question 23.
A student collected common names and formulae of some substances but he forgot to note which formula is for which compound. Help him to match the correct formula. (Board Term I, 2013)
| (i) Caustic soda | NaHCO3. |
| (ii) Slaked lime | CaO |
| (iii) Baking soda | NaOH |
| (iv) Lime | Ca(OH)2. |
Answer:
(i) Caustic soda → NaOH
(ii) Slaked lime → Ca(OH)2
(iii) Baking soda → NaHCO3
(iv) Lime → CaO
Question 24.
List the important products of the Chlor-alkali process. Write one important use of each. (2020)
Answer:
Sodium hydroxide is prepared by electrolysis of an aqueous solution of sodium chloride (brine). The complete reaction can be represented as: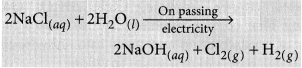
The process of electrolysis of sodium chloride solution is called chlor-alkali process because of the products formed : chlor for chlorine and alkali for sodium hydroxide. The three very useful products obtained by the electrolysis of sodium chloride solution are sodium hydroxide, chlorine and hydrogen.
At anode : Cl2 gas is liberated At cathode : H2 gas is liberated.
Uses of sodium hydroxide: In the manufacture of soaps and detergents.
Uses of chlorine : As a germicide and disinfectant for sterilisation of drinking water and for water of swimming pools.
Uses of hydrogen: In the manufacture of ammonia which is used for the preparation of various fertilizers like urea, ammonium sulphate etc.
Question 25.
How is washing soda prepared from sodium carbonate? Give its chemical equation. State the type of this salt. Name the type of hardness of water which can be removed by it? (2020)
Answer:
Washing soda is prepared by recrystallisation of sodium carbonate: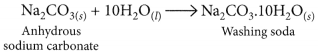
It is used to remove the permanent hardness of water. Hard water is treated with a calculated amount of washing soda when chlorides and sulphates of calcium and magnesium present in hard water get precipitated as insoluble calcium and magnesium carbonates which can be easily filtered off. The water thus becomes soft.
CaCl2 + Na2CO3 → CaCO3↓ + 2NaCl
MgSO4 + Na2CO3 → MgCOsub>3↓ + Na2SO4
Question 26.
Give reasons for the following:
(i) Only one half of water molecule is shown in the formula of plaster of Paris.
(ii) Sodium hydrogen carbonate is used as an antacid.
(iii) On strong heating, blue coloured copper sulphate crystals turn white. (2020)
Answer:
(i) Only one half of water molecule is shown in the formula of plaster of Paris (CaSO4.
(ii) Acidity can be neutralised by a base. Sodium hydrogen carbonate can be used as an antacid solution because it is a weak base and will react
with excess acid produced in the stomach due to hyperacidity and will neutralise it.
(iii) Blue coloured copper sulphate crystals are hydrated copper sulphate, CuSO4.5H2O. On heating blue copper sulphate crystals looses its water of crystallisation and turns into anhydrous copper sulphate which is white in colour.![]()
Question 27.
During electrolysis of brine, a gas ‘G’ is liberated at anode. When this gas ‘G’ is passed through slaked lime, a compound ‘C’ is formed, which is used for disinfecting drinking water.
(i) Write formula of ‘G’ and ‘C’.
(ii) State the chemical equations involved.
(iii) What is common name of compound ‘C’ ? Give its chemical name. (2020)
Answer:
(i) During electrolysis of brine, chlorine is obtained at anode. When chlorine is passed through slaked lime, bleaching powder is formed which is used for disinfecting drinking water. Hence, G is Cl2 and C is CaOCl2.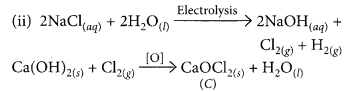
(iii) Common name of C is bleaching powder. Its chemical name is calcium hypochlorite.
Question 28.
Identify the acid and the base from which sodium chloride is obtained. Which type of salt is it? When is it called rock salt? How is rock salt formed? (Delhi 2019)
Answer:
Sodium chloride is obtained by the neutralisation of sodium hydroxide (base) with hydrochloric acid (acid). It is a neutral salt. Common salt found in the form of solid deposits is often brown in colour due to presence of impurities which is called rock salt. Rock salt is formed by evaporation of salty water of inland lakes.
Question 29.
A white powder is added while baking cakes to make it soft and spongy. Name its main ingredients. Explain the function of each ingredient. Write the chemical reaction taking place when the powder is heated during baking. (AI2019)
Answer:
The white powder added while baking cakes to make it soft and spongy is baking powder. Its main ingredients are sodium hydrogen carbonate and a mild edible acid like tartaric acid or citric acid. NaHCO3 decomposes to give out CO2 which causes the cake to rise and makes it soft and spongy. The function of tartaric acid or citric acid is to neutralise sodium carbonate formed during heating which can otherwise make the cake bitter. Reaction taking place when the powder is heated:![]()
Question 30.
The pH of a salt used to make tasty and crispy pakoras is 14. Identify the salt and write a chemical equation for its formation. List its two uses. (2018)
Answer:
Salt used to make tasty and crispy pakoras is sodium bicarbonate (NaHCO3), pH = 9. On large scale, sodium bicarbonate is prepared as: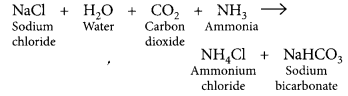
Two uses of sodium bicarbonate are as follows :
(i) It is used as an antacid in medicines.
(ii) It is used as an additive in food and drinks.
Note : In the question paper, the given pH is 14 which should be 9.
Question 31.
Write one point of difference between each of the following:
(i) A hydrated salt and an anhydrous salt.
(ii) Washing soda and soda ash.
(iii) Baking soda and baking powder. (Board Term 1,2017)
Answer:
(i)
| Hydrated salt | Anhydrous salt |
| A salt with one or more chemically combined water molecule is called hydrated salt, e.g., washing soda, Na2CO3.10H2.O | A salt in which all water molecules are removed, is called anhydrous salt, e.g., soda ash, Na2.CO3. |
(ii)
| Washing soda | Soda ash |
| The hydrated salt of sodium carbonate containing 10 molecules of water of crystallisation, is known as washing soda i.e., Na2CO3.10H2.O. | The anhydrous sodium carbonate (Na2CO3.) which does not contain water of crystallisation, is known as soda ash. |
(iii)
| Baking soda | Baking powder |
| Baking soda is sodiumhydrogen carbonate with the formula, NaHCO3. | Baking powder is a mixture of NaHCO3. and tartaric acid or citric acid. |
Question 32.
Complete the following table:
| Sample Solution | Red litmus solution | Blue litmus solution | Phenolp-hthalein solution |
| Acetic acid | |||
| Sodium hydroxide | |||
| Baking soda |
Answer:
| Sample solution | Red litmus solution | Blue | Phenolp- |
| Acetic acid (CH3.COOH) | No effect | litmus | hthalein |
| Sodium hydroxide (NaOH) | Blue | solution | solution |
| Baking Soda (NaHCO3.) | Blue | Red | Colourless |
Question 33.
A white coloured powder is used by doctors for supporting fractured bones.
(a) Write chemical name and formula of the powder.
(b) When this white powder is mixed with water a hard solid mass is obtained. Write balanced chemical equation for this change. (Board Term I, 2016)
Answer:
(a) Chemical name of the powder is calcium sulphate hemihydrate. Chemical formula of the powder is CaSO4.
(b) When water is added to plaster of Paris, it sets into a hard mass in about half an hour. The setting of plaster of Paris is due to its hydration to form crystals of gypsum which set to form a hard, solid mass.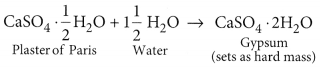
Question 34.
(a) Define an acid-base indicator. Mention one synthetic acid-base indicator.
(b) If someone in the family is suffering from a problem of acidity after overeating, which of the following substances would you suggest as a remedy?
Lemon juice, vinegar or baking soda solution. Mention the property on the basis of which you will choose the remedy. (Board Term I, 2014)
Answer:
(a) Acid – base indicators : The indicators which show different colours in acidic and basic medium are called acid-base indicators. Phenolphthalein is a synthetic indicator.
(b) Acidity can be neutralised by a base. Hence, we should choose baking soda solution because it is a weak base and will react with excess acid produced in the stomach due to hyperacidity and will neutralise it.
Question 35.
Define water of crystallisation. Give the chemical formula for two compounds as examples. How can it be proved that the water of crystallisation makes a difference in the state and colour of the compounds? (2020)
Answer:
Water of crystallisation : It is the fixed number of water molecules present in one formula unit of a salt, e.g., Gypsum (CaSO4.2H2O) has two molecules of water of crystallisation.
In hydrated copper sulphate (CuSO4.5H2O), there are five molecules of water of crystallisation.
Activity:
– Take few crystals of copper sulphate in a dry boiling tube. These are blue in colour.
– Heat the boiling tube by holding it with a test tube holder on the flame of the burner.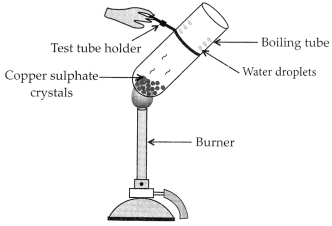
Observations : You will observe that the colour of copper sulphate crystals after heating becomes white. You may also notice water droplets on the mouth side of the boiling tube which are obtained from water of crystallisation.After adding 2-3 drops of water on the white sample of copper sulphate (obtained after heating) you will observe that the blue colour of copper sulphate crystals is restored.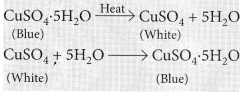
Question 36.
(a) A student dropped a few pieces of marble in dilute hydrochloric acid contained in a test tube. The evolved gas was passed through lime water. What change would be observed in lime water? Write balanced chemical equations for both the changes observed.
(b) State the chemical property in each case on which the following uses of baking soda are based:
(i) as an antacid
(ii) as a constituent of baking powder. (Board Term I, 2017)
Answer:
(a) When marble reacts with dilute HCl carbon dioxide gas is liberated.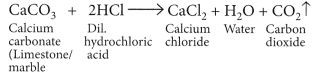
When CO2 gas is passed through lime water, insoluble calcium carbonate is formed which appears milky.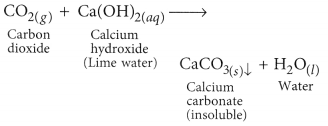
(b) (i) The excess acid formed in the stomach due to various reasons (one being overeating) is neutralised by sodium hydrogen carbonate. Hence, it is used as an ingredient of antacid.
(ii) Baking soda (sodium hydrogen carbonate) is a constituent of baking power. On heating it gives out CO2 which causes the cake to rise and make it soft and spongy.
Question 37.
(a) What are anhydrous and hydrated salts? Explain with a suitable example of each]
(b) How is plaster of Paris prepared? What reaction takes place when it sets to a hard mass? (Board Term I, 2017)
Answer:
(a) Refer to answer 31(i).
(b) It is prepared from gypsum which is calcium sulphate dihydrate (CaSO4.2H2O). Gypsum is heated in a kiln to a temperature of 100°C (373 K). At this temperature, it loses three-fourth of its water of crystallisation forming plaster of Paris.
Refer to answer 33(b).
Question 38.
(a) Write the chemical formula of hydrated copper sulphate and anhydrous copper sulphate. Giving an activity illustrate how these two are interconvertible.
(b) Write chemical names and formulae of plaster of Paris and gypsum. (Board Term 1, 2016)
Answer:
(a) The chemical formula of hydrated copper sulphate is CuSO4.5H2O(s) and anhydrous copper sulphate is CuSO4(s).
For activity refer to answer 35.
(b) Plaster of Paris is calcium sulphate hemihydrate; CaSO4.
(CaSO4.2H2O).
Question 39.
How is sodium hydroxide produced? Write the balanced chemical equation also. Why is this process called as chlor-alkali process? In this process name the products given off at:
(a) anode
(b) cathode
Write one use of each of these products. (Board Term I, 2015)
Answer:
Refer to answer 24.
Question 40.
What is water of crystallization? Write the common name and chemical formula of a commercially important compound which has ten water molecules as water of crystallization. How is this compound obtained? Write the chemical equation also. List any two uses of this compound. (Board Term I, 2015)
Answer:
Water of crystallization : Crystals of some salts contain certain amount of associated water.
The water associated with the crystal (or molecule) of any salt is called water of crystallisation.
The hydrated salt is known as washing soda which is sodium carbonate containing 10 molecules of water of crystallization, i.e., it is sodium carbonate decahydrate. Its molecular formula is Na2CO3.10H2O.
It can be obtained by heating baking soda followed by recrystallisation from its aqueous solution.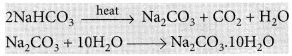
Uses of sodium carbonate:
(i) For the manufacture of glass, soap, papers and chemicals like caustic soda (NaOH), borax, etc.
(ii) For washing purposes (laundry works).
Question 41.
(a) Name and describe giving chemical equation the process used for producing sodium hydroxide. Why is this process so named?
(b) Give one use of each of any two products obtained in this process. (Board Term I, 2014)
Answer:
Refer to answer 24.
Question 42.
(a) You have three solutions – A, B and C having a pH of 6, 2 and 9 respectively. Arrange these solutions in increasing order of hydrogen ion concentration. Which of the three is most acidic? What happens to the hydrogen ion concentration in A as it is diluted?
(b) If someone is suffering from a stomach problem called acidity, why is a solution of baking soda offered as a remedy?
(c) Write chemical name and formula of baking soda. (Board Term I, 2013)
Answer:
(a) The solution having lower pH will have more hydrogen ion concentration. Hence, solution B (i.e., pH = 2) will have more hydrogen ion concentration.![]()
Solution B is most acidic.
Adding water to solution A, will reduce the concentration of hydrogen ions in the solution.
(b) Refer to answer 26(ii).
(c) Refer to answer 31(iii).
CBSE Class 10 Science Notes Chapter 2 Acids Bases and Salts
Indicators: Indicators are substances which indicate the acidic or basic nature of the solution by the colour change.
Types of Indicator: There are many types of indicators. Some common types of indicators are:
1. Natural Indicators: Indicators obtained from natural sources are called Natural Indicators. Litmus, turmeric, red cabbage, China rose, etc., are some common natural indicators used widely to show the acidic or basic character of substances.
Litmus: Litmus is obtained from lichens. The solution of litmus is purple in colour. Litmus paper comes in two colours- blue and red.
An acid turns blue litmus paper red.
A base turns red litmus paper blue.
Turmeric: Turmeric is another natural indicator. Turmeric is yellow in colour. Turmeric solution or paper turns reddish brown with base. Turmeric does not change colour with acid.
Red Cabbage: The juice of red cabbage is originally purple in colour. Juice of red cabbage turns reddish with acid and turns greenish with base.
2. Olfactory Indicator: Substances which change their smell when mixed with acid or base are known as Olfactory Indicators. For example; Onion, vanilla etc.
Onion: Paste or juice of onion loses its smell when added with base. It does not change its smell with acid.
Vanilla: The smell of vanilla vanishes with base, but its smell does not vanish with an acid.
Olfactory Indicators are used to ensure the participation of visually impaired students in the laboratory.
3. Synthetic Indicator: Indicators that are synthesized in the laboratory are known as Synthetic Indicators. For example; Phenolphthalein, methyl orange, etc.
Phenolphthalein is a colourless liquid. It remains colourless with acid but turns into pink with a base.
Methyl orange is originally orange in colour. It turns into the red with acid and turns into yellow with base.
| Indicator | Original Colour | Acid | Base |
| Red litmus | Red | No Change | Blue |
| Blue litmus | Blue | Red | No change |
| Turmeric | Yellow | No Change | Reddish brown |
| Red cabbage juice | Purple | Reddish | Greenish yellow |
| Phenolphthalein | Colourless | Colourless | Pink |
| Methyl Orange | Orange | Red | Yellow |
| Onion | n/a | No change | Smell vanishes |
| Vanilla | n/a | No change | Smell vanishes |
Acids: Acids are sour in taste, turn blue litmus red, and dissolve in water to release H+ ions.
Example: Sulphuric acid (H2SO4), Acetic Acid (CH3COOH), Nitric Acid (HNO3) etc.
Properties of Acids:
- Acids have a sour taste.
- Turns blue litmus red.
- Acid solution conducts electricity.
- Release H+ ions in aqueous solution.
Types of Acids: Acids are divided into two types on the basis of their occurrence i.e., Natural acids and Mineral acids.
(i) Natural Acids: Acids which are obtained from natural sources are called Natural Acids or Organic Acids.
Examples:
Methanoic acid (HCOOH)
Acetic acid (CH3COOH)
Oxalic acid (C2H2O4) etc.
| Organic Acids and their Sources | |
| Acids | Sources |
| Acetic acid | Vinegar |
| Ascorbic acid | Guava, amla |
| Citric acid | Lemon, orange and other citrus fruits |
| Lactic acid | Sour milk, curd |
| Methanoic acid | Ant sting, nettle sting |
| Oxalic acid | Tomato |
| Tartaric acid | Tamarind |
(ii) Mineral Acids: Acids that are prepared from minerals are known as Mineral Acids Example; Inorganic acids, man-made acids or synthetic acid are also known as Mineral Acids.
Example:
Hydrochloric acid (HCl)
Sulphuric acid (H2SO4)
Nitric acid (HNO3)
Carbonic acid (H2CO3)
Phosphoric acid (H3PO4) etc.
Chemical Properties of Acid:
(i) Reaction of acids with metal: Acids give hydrogen gas along with respective salt when they react with a metal.
Metal + Acid → Salt + Hydrogen
Examples:
Hydrogen gas and zinc chloride are formed when hydrochloric acid reacts with zinc metal.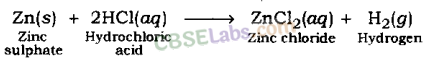
Hydrogen gas and sodium sulphate are formed when sulphuric acid reacts with sodium metal.
Test For Hydrogen Gas: The gas evolved after reaction of acid with metal can be tested by bringing a lighted candle near it. If the gas bums with a pop sound, then it confirms the evolution of hydrogen gas. Burning with pop sound is the characteristic test for hydrogen gas.
(ii) Reaction of acids with metal carbonate: Acids give carbon dioxide gas and respective salts along with water when they react with metal carbonates.
Metal carbonate + Acid → Salt + Carbon dioxide + Water
Examples:
Hydrochloric acid gives carbon dioxide gas, sodium chloride along with water when reacts with sodium carbonate.
Sulphuric acid gives calcium sulphate, carbon dioxide gas, calcium sulphate and water when it reacts with calcium carbonate
Nitric acid gives sodium nitrate, water and carbon dioxide gas when it reacts with sodium carbonate.
(iii) Reaction of acid with hydrogen carbonates (bicarbonates): Acids give carbon dioxide gas, respective salt and water when they react with metal hydrogen carbonate.
Acid + Metal hydrogen carbonate → Salt + Carbon dioxide + Water
Example:
Sulphuric acid gives sodium sulphate, Carbon dioxide gas and water when it reacts with sodium bicarbonate.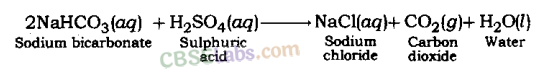
Test For Evolution of Carbon Dioxide Gas: Carbon dioxide turns lime water milky when passed through it. This is the characteristic test for carbon dioxide gas.
The gas evolved because of reaction of the acid with metal carbonate or metal hydrogen carbonate turns lime water milky. This shows that the gas is carbon dioxide gas. This happens because of the formation of a white precipitate of calcium carbonate.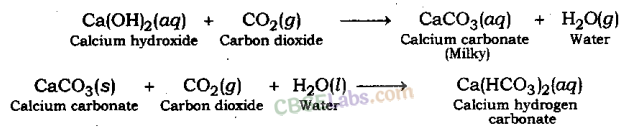
But when excess of carbon dioxide is passed through lime water, it makes milky colour of lime water disappear. This happens because of formation of calcium hydrogen carbonate. As calcium hydrogen carbonate is soluble in water, thus, the milky colour of solution mixture disappears.
Common in Acids: Acids give hydrogen gas when they react with metal. This shows that all acids contains hydrogen. For example; Hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3), etc.
When an acid is dissolved in water, it dissociates hydrogen. The dissociation of hydrogen ion in aqueous solution is the common property in all acids. Because of the dissociation of hydrogen ion in aqueous solution, an acid shows acidic behaviour.
Examples:
Hydrochloric acid (HCl) gives hydrogen ion (H+) and chloride ion (Cl–) when it is dissolved in water.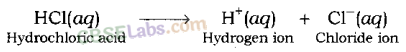
Acetic acid (CH3COOH) gives acetate ion (CH3COO–) and hydrogen ion (H+).
Acids
Strong Acids
An acid which is completely ionised in water and produces (H+) is called Strong Acid.
Examples: Hydrochloric acid (HCl), Sulphuric acid (H2SO4), Nitric acid (HNO3)
Weak Acids
An acid which is partially ionised in water and thus produces a small amount of hydrogen ions (H+) is called a Weak Acid.
Example: Acetic acid (CH3COOH), Carbonic acid (H2CO3)
When a concentrated solution of acid is diluted by mixing water, then the concentration of Hydrogen ions (H+) or hydronium ion (H3O–) per unit volume decreases.
Bases: Bases are bitter in taste, have soapy touch, turn red litmus blue and give hydroxide ions (OH–) in aqueous solution.
Examples: Sodium hydroxide (caustic soda) – NaOH
Calcium hydroxide – Ca(OH)2
Potassium hydroxide (caustic potash) – (KOH)
Properties of Bases:
- Have a bitter taste.
- Soapy to touch.
- Turns red litmus blue.
- Conducts electricity in solution.
- Release OH– ions in Aqueous Solution
Types of bases: Bases can be divided in two types – Water soluble and Water-insoluble.
The hydroxide of alkali and alkaline earth metals are soluble in water. These are also known as alkali.
For example; sodium hydroxide, magnesium hydroxide, calcium hydroxide, etc. Alkali is considered a strong base.
Chemical properties of bases:
(i) Reaction of Base with Metals: When alkali (base) reacts with metal, it produces salt and hydrogen gas.
Alkali + Metal → Salt + Hydrogen
Examples: Sodium hydroxide gives hydrogen gas and sodium zincate when reacts with zinc metal.
Sodium aluminate and hydrogen gas are formed when sodium hydroxide reacts with aluminium metal.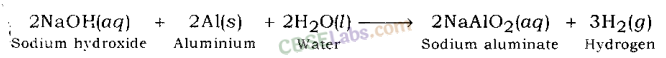
(ii) Reaction of Base with Oxides of Non-metals: Non-metal oxides are acidic in nature. For example; carbon dioxide is a non-metal oxide. When carbon dioxide is dissolved in water it produces carbonic acid.
Therefore, when a base reacts with non-metal oxide, both neutralize each other resulting respective salt and water.
Base + Non-metal oxide → Salt + Water
(Non-metal oxides are acidic in nature)
Examples:
Sodium hydroxide gives sodium carbonate and water when it reacts with carbon dioxide.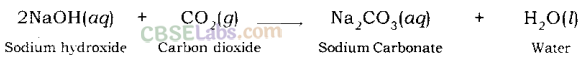
Calcium hydroxide gives calcium carbonate and water when it reacts with carbon dioxide.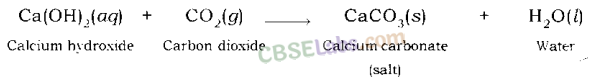
(iii) Neutralisation Reaction: An acid neutralizes a base when they react with each other and respective salt and water are formed.
Acid + Base → Salt + Water
Since, the reaction between acid and base both neutralize each other, hence, it is also known as Neutralization Reaction.
Examples: Sodium chloride and water are formed when hydrochloric acid reacts with sodium hydroxide (a strong base).
In a similar way, calcium chloride is formed along with water when hydrochloric acid reacts with calcium hydroxide (a base).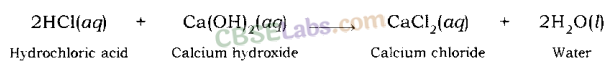
(iv) Reaction of Acid with Metal Oxides: Metal oxides are basic in nature. Thus, when an acid reacts with a metal oxide both neutralize each other. In this reaction, the respective salt and water are formed.
Acid + Metal Oxide → Salt + Water
(Metal oxides are basic in nature)
Examples:
Calcium is a metal, thus, calcium oxide is a metallic oxide which is basic in nature. When an acid, such as hydrochloric acid, reacts with calcium oxide, neutralization reaction takes place and calcium chloride, along with water is formed.
Similarly, when sulphuric acid reacts with zinc oxide, zinc sulphate and water are formed.
Common in all bases: A base dissociates hydroxide ion in water, which is responsible for the basic behaviour of a compound.
Example: When sodium hydroxide is dissolved in water, it dissociates hydroxide ion and sodium ion.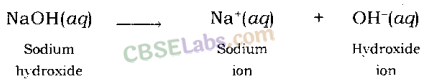
Similarly, when potassium hydroxide is dissolved in water, it dissociates hydroxide ion and potassium ion.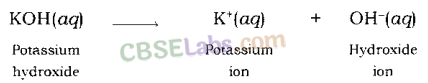
Thus, the base shows its basic character because of dissociation of hydroxide ion.
Neutralisation Reaction: When an acid reacts with a base, the hydrogen ion of acid combines with the hydroxide ion of base and forms water. As these ions combine together and form water instead of remaining free, thus, both neutralize each other.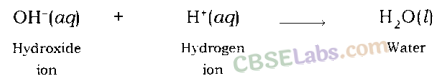
Example: When sodium hydroxide (a base) reacts with hydrochloric acid, sodium hydroxide breaks into a sodium ion and hydroxide ion and hydrochloric acid breaks into hydrogen ion and chloride ion.
Hydrogen ion and hydroxide ion combine together and form water, while sodium ion and chloride ion combine together and form sodium chloride.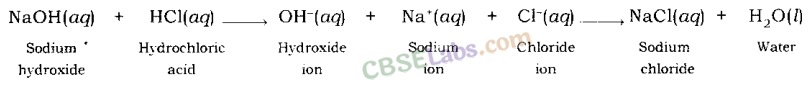
Dilution of Acid and Base: The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows the concentration of acid or base.
By mixing of acid to water, the concentration of hydrogen ion per unit volume decreases. Similarly, by addition of base to water, the concentration of hydroxide ion per unit volume decreases. This process of addition of acid or base to water is called Dilution and the acid or base is called Diluted.
The dilution of acid or base is exothermic. Thus, acid or base is always added to water and water is never added to acid or base. If water is added to a concentrated acid or base, a lot of heat is generated, which may cause splashing out of acid or base and may cause severe damage as concentrated acid and base are highly corrosive.
Strength of Acid and Base: Acids in which complete dissociation of hydrogen ion takes place are called Strong Acids. Similarly, bases in which complete dissociation of hydroxide ion takes place are called Strong Bases.
In mineral acid, such as hydrochloric acid, sulphuric acid, nitric acid, etc. hydrogen ion dissociates completely and hence, they are considered as strong acids. Since inorganic acids hydrogen ions do not dissociate completely, so they are weak acids.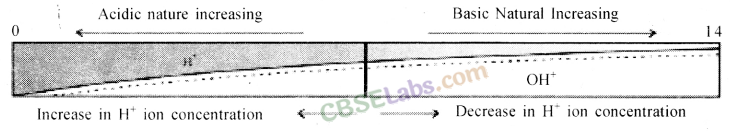
For water or neutral solutions : pH = 7
For acidic solutions : pH < 7
For basic solution : pH > 7
Universal Indicator: Using a litmus paper, phenolphthalein, methyl orange, etc. only the acidic or basic character of a solution can be determined, but the use of these indicators does not give the idea about the strength of acid or base. So, to get the strength as well as acidic and basic nature of a given solution universal indicator is used.
Universal indicator shows different colour over the range of pH value from 1 to 14 for a given solution. Universal indicator is available both in the form of strips and solution. Universal indicator is the combination of many indicators, such as water, propanol, phenolphthalein, sodium salt, sodium hydroxide, methyl red, bromothymol blue monosodium salt, and thymol blue monosodium salt. The colour matching chart is supplied with a universal indicator which shows the different colours for different values of pH.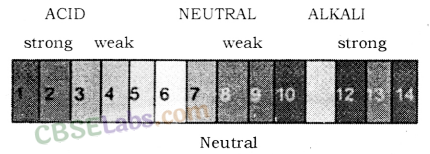
pH value shown by different colours role of pH everyday life:
(i) pH in our digestive system: Dilute HCl (Hydrochloric acid) helps in digestion of food (proteins) in our stomach. Excess acid in stomach causes acidity (indigestion). Antacids like magnesium hydroxide [Mg(OH)2] also known as milk of magnesia and sodium hydrogen carbonate (baking soda) are used to neutralize excess acid.
(ii) Tooth decay caused by acids: The bacteria present in our mouth converts the sugar into acids. When the pH of acid formed in the mouth falls below 5.5, tooth-decaying starts. The excess acid has to be removed by cleaning the teeth with a good quality toothpaste because these kinds of toothpaste are alkaline in nature.
(iii) Soil of pH and plant growth: Most of the plants have a healthy growth when the soil has a specific pH (close to 7) range which should be neither alkaline nor highly acidic. Therefore,
- Compound ‘X’ is Sodium hydroxide (NaOH).
- Compound ‘A’ is Zinc sulphate (ZnSO4).
- Compound ‘B’ is Sodium chloride (NaCl).
- Compound ‘C’ is Sodium acetate (CH3COONa)
Salts: Salts are the ionic compounds which are produced after the neutralization reaction between acid and base. Salts are electrically neutral. There are number of salts but sodium chloride is the most common among them. Sodium chloride is also known as table salt or common salt. Sodium chloride is used to enhance the taste of food.
Characteristics of salt:
- Most of the salts are crystalline soild.
- Salts may be transparent or opaque.
- Most of the salts are soluble in water.
- Solution of the salts conducts electricity in their molten state also.
- The salt may be salty, sour, sweet, bitter and umami (savoury).
- Neutral salts are odourless.
- Salts can be colourless or coloured.
Family of Salt: Salts having common acidic or basic radicals are said to belong to the same family.
Example:
(i) Sodium chloride (NaCl) and Calcium chloride (CaCl2) belongs to chloride family.
(ii) Calcium chloride (CaCl2) and Calcium sulphate (CaSO4) belongs to calcium family.
(iii) Zinc chloride (ZnCl2) and Zinc sulphate (ZnSO4) belongs to the zinc family.
Neutral, Acidic and Basic Salts:
(i) Neutral Salt: Salts produced because of reaction between a strong acid and strong base are neutral in nature. The pH value of such salts is equal to 7, i.e. neutral.
Example : Sodium chloride, Sodium sulphate. Postassium chloride, etc.
Sodium chloride (NaCl): It is formed after the reaction between hydrochloric acid (a strong acid) and sodium hydroxide (a strong base).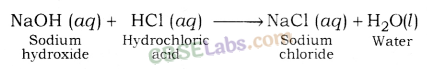
Sodium Sulphate (Na2SO4): It is formed after the reaction between sodium hydroxide (a strong base) and sulphuric acid ( a strong acid).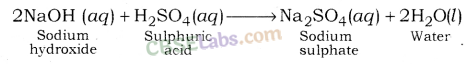
Potassium Chloride (KCl): It is formed after the reaction between potassium hydroxide (a strong base) and hydrochloric acid (a strong acid).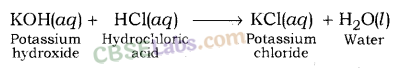
(ii) Acidic Salts: Salts which are formed after the reaction between a strong acid and weak base are called Acidic salts. The pH value of acidic salt is lower than 7. For example Ammonium sulphate, Ammonium chloride, etc.
Ammonium chloride is formed after reaction between hydrochloric acid (a strong acid) and ammonium hydroxide (a weak base).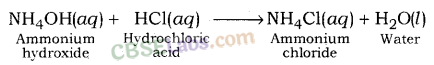
Ammonium sulphate is formed after reaction between ammonium hydroxide (a weak base) and sulphuric acid (a strong acid).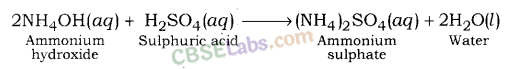
(iii) Basic Salts: Salts which are formed after the reaction between a weak acid and strong base are called Basic Salts. For example; Sodium carbonate, Sodium acetate, etc.
Sodium carbonate is formed after the reaction between sodium hydroxide (a strong base) and carbonic acid (a weak acid).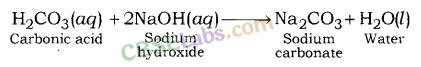
Sodium acetate is formed after the reaction between a strong base, sodium hydroxide (a strong base) and acetic acid, (a weak acid).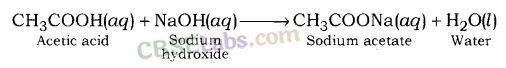
Cause of formation of acidic, basic and neutral salts:
- When a strong acid reacts with a weak base, the base is unable to fully neutralize the acid. Due to this, an acidic salt is formed.
- When a strong base reacts with a weak acid, the acid is unable to fully neutralize the base. Due to this, a basic salt is formed.
- When equally strong acid and a base react, they fully neutralize each other. Due to this, a neutral salt is formed.
pH value of salt:
- Neutral salt: The pH value of a neutral salt is almost equal to 7.
- Acidic salt: The pH value of an acidic salt is less than 7.
- Basic salt: The pH value of a basic salt is more than 7.
Some Important Chemical Compounds
1. Common Salt (Sodium Chloride): Sodium chloride (NaCl) is also known as Common or Table Salt. It is formed after the reaction between sodium hydroxide and hydrochloric acid. It is a neutral salt. The pH value of sodium chloride is about 7. Sodium chloride is used to enhance the taste of food. Sodium chloride is used in the manufacturing of many chemicals.
Important chemical from sodium chloride
Sodium Hydroxide (NaOH): Sodium hydroxide is a strong base. It is also known as caustic soda. It is obtained by the electrolytic decomposition of solution of sodium chloride (brine). In the process of electrolytic decomposition of brine (aqueous solution of sodium chloride), brine decomposes to form sodium hydroxide. In this process, chlorine is obtained at anode and hydrogen gas is obtained at cathode as by products. This whole process is known as Chlor – Alkali process.
Use of products after the electrolysis of brine:
- Hydrogen gas is used as fuel, margarine, in making of ammonia for fertilizer, etc.
- Chlorine gas is used in water treatment, manufacturing of PVC, disinfectants, CFC, pesticides. It is also used in the manufacturing of bleaching powder and hydrochloric acid.
- Sodium hydroxide is used for degreasing of metals, manufacturing of paper, soap, detergents, artificial fibres, bleach, etc.
2. Bleaching Powder (CaOCl2): Bleaching powder is also known as chloride of lime. It is a solid and yellowish white in colour. Bleaching powder can be easily identified by the strong smell of chlorine.
When calcium hydroxide (slaked lime) reacts with chlorine, it gives calcium oxychloride (bleaching powder) and water is formed.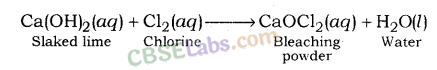
Aqueous solution of bleaching powder is basic in nature. The term bleach means removal of colour. Bleaching powder is often used as bleaching agent. It works because of oxidation. Chlorine in the bleaching powder is responsible for bleaching effect.
Use of Bleaching Powder:
- Bleaching powder is used as disinfectant to clean water, moss remover, weed killers, etc.
- Bleaching powder is used for bleaching of cotton in textile industry, bleaching of wood pulp in paper industry.
- Bleaching powder is used as oxidizing agent in many industries, such as textiles industry, paper industry, etc.
3. Baking Soda (NaHCO3): Baking soda is another important product which can be obtained using byproducts of chlor – alkali process. The chemical name of baking soda is sodium hydrogen carbonate (NaHCO3) or sodium bicarbonate. Bread soda, cooking soda, bicarbonate of soda, sodium bicarb, bicarb of soda or simply bicarb, etc. are some other names of baking soda.
Preparation Method: Baking soda is obtained by the reaction of brine with carbon dioxide and ammonia. This is known as Solvay process.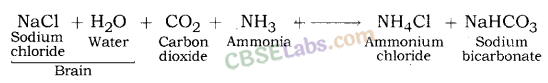
In this process, calcium carbonate is used as the source of CO2 and the resultant calcium oxide is used to recover ammonia from ammonium chloride.
Properties of Sodium Bicarbonate:
- Sodium bicarbonate is white crystalline solid, but it appears as fine powder.
- Sodium hydrogen carbonate is amphoteric in nature.
- Sodium hydrogen carbonate is sparingly soluble in water.
- Thermal decomposition of sodium hydrogen carbonate (baking soda).
- When baking soda is heated, it decomposes into sodium carbonate, carbon dioxide and water.
2NaHCO3 + heat → Na2CO3 + CO2 + H2O - Sodium carbonate formed after thermal decomposition of sodium hydrogen carbonate decomposes into sodium oxide and carbon dioxide on further heating.
Na2CO3 → Na2O + CO2
This reaction is known as Dehydration reaction.
Use of Baking Soda:
- Baking soda is used in making of baking powder, which is used in cooking as it produces carbon dioxide which makes the batter soft and spongy.
- Baking soda is used as an antacid.
- Baking soda is used in toothpaste which makes the teeth white and plaque free.
- Baking soda is used in cleansing of ornaments made of silver.
- Since sodium hydrogen carbonate gives carbon dioxide and sodium oxide on strong heating, thus, it, is used as a fire extinguisher.
Baking Powder: Baking powder produces carbon dioxide on heating, so it is used in cooking to make the batter spongy. Although, baking soda also produces carbon dioxide on heating, but it is not used in cooking because on heating, baking soda produces sodium carbonate along with carbon dioxide. The sodium carbonate, thus, produced, makes the taste bitter.
Baking powder is the mixture of baking soda and a mild edible acid. Generally, tartaric acid is mixed with baking soda to make baking powder.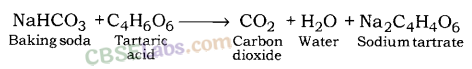
When baking powder is heated, sodium hydrogen carbonate (NaHCO3) decomposes to give CO2 and sodium carbonate (Na2CO3). CO2 causes bread and cake fluffy. Tartaric acid helps to remove bitter taste due to formation of Na2CO3.
4. Washing Soda (Sodium Carbonate)
Preparation Method: Sodium carbonate is manufactured by the thermal decomposition of sodium hydrogen carbonate obtained by Solvay process.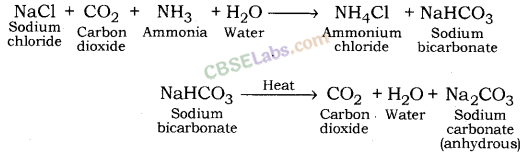
The sodium carbonate obtained in this process is dry. It is called Soda ash or Anhydrous sodium carbonate. Washing soda is obtained by rehydration of anhydrous sodium carbonate.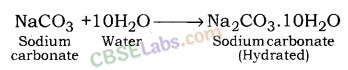
Since there are 10 water molecules in washing soda, hence, it is known as Sodium Bicarbonate Decahydrate.
Sodium carbonate is a crystalline solid and it is soluble in water when most of the carbonates are insoluble in water.
Use of sodium carbonate:
- It is used in the cleaning of cloths, especially in rural areas.
- In the making of detergent cake and powder.
- In removing the permanent hardness of water.
- It is used in glass and paper industries.
The water of Crystallization: Many salts contain water molecule and are known as Hydrated Salts. The water molecule present in salt is known as Water of crystallization.
Examples:
Copper sulphate pentahydrate (CuSO4.5H2O): Blue colour of copper sulphate is due to presence of 5 molecules of water. When copper sulphate is heated, it loses water molecules and turns: into grey – white colour, which is known as anhydrous copper sulphate. After adding water, anhydrous copper sulphate becomes blue again.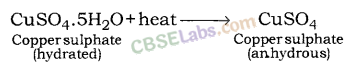
Acids: Substances which turn blue litmus solution red are called acids. Acids are sour in taste.
Bases: Substances which change red litmus solution blue are called bases. They are bitter in taste.
Mineral Acids: Acids which are obtained from minerals like sulphates, nitrates, chlorides etc. are called mineral acids, example, H2SO4 (Sulphuric acid), HNO3 (Nitric acid) and HCl (Hydrochloric acid).
Organic Acids: Acids which are obtained from plants and animals are called organic acids. Example citric acid, ascorbic acid, tartaric acid, lactic acid, acetic acid.
Hydronium Ions: They are formed by the reaction of H+ (from acid) and H2O. It is because H+ is unstable.
Universal Indicator: A universal indicator is a mixture of indicators which shows a gradual but well-marked series of colour changes over a very wide range of change in concentration of H+ ions.
Strong Acids: Acids which dissociate into ions completely are called strong acids. Example, H2SO4, HCl.
Weak Acids: Acids which do not dissociate into ions completely are called weak acids. Example, citric acid, acetic acid.
Chemical Properties of Acids:
- Acids react with active metals to give salt and hydrogen gas.
- Acids react with metal carbonates and metal hydrogen carbonates to give salt, water and carbon dioxide.
- Acids react with bases to give salt and water. This reaction is called a neutralization reaction.
- Acids react with metal oxides to give salt and water.
Chemical Properties of Bases:
- Reaction with metals: Certain metals such as zinc, aluminium and tin react with alkali solutions on heating and hydrogen gas is evolved.
- Reaction with acids: Bases react with acids to form salt and water.
Indicators: Indicators are substances which indicate the acidic or basic nature of the solution by their colour change.
pH Scale: A scale for measuring hydrogen ion concentration in a solution.
The pH of a solution is defined as the negative logarithm of hydrogen ion concentration in moles per litre.
pH = -log [H+]
pH = -log [H3O+]
where [H+] or [H3O+] represents concentrations of hydrogen ions in a solution.
- The pH of a neutral solution is 7.
- The pH of an acidic solution is < 7.
- The pH of a basic solution is > 7.
Some Important Compounds and their Uses: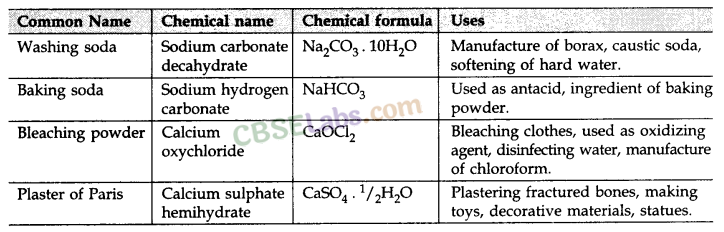
Equations of Acids, Bases and Salts:
- Acid + Metal → Salt + Hydrogen gas
H2SO4 + Zn → ZnSO4 + H2 - Base + Metal → Salt + Hydrogen gas
2NaOH + Zn → Na2ZnO2 (Sodium zincate) + H2 - Base + Acid → Salt + Water
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l) - Acids give hydronium ions in water
HCl + H2O → H3O+ + Cl– - Bases generate OH- ions in water
NaOH (aq) + H2O → Na+ (aq) + O– (aq)
Reactions Of Important Chemical Compounds:
- Preparation of Bleaching powder: By the action of chlorine on dry slaked lime
Ca(OH)2 + Cl2 → CaOCl2 + H2O - On heating, baking soda liberates CO2
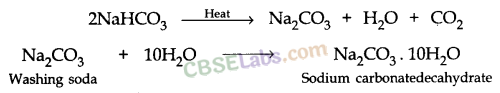
- Preparation of Plaster of Paris:
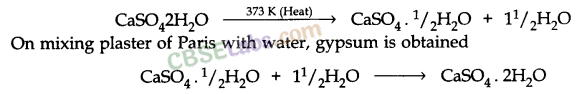
NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases And Salts
Short Answer Type Questions
1.Match the acids given in Column (A) with their correct source given in Column (B).
Ans.(a) —> (iv) (b) —> (iii) (c) —> (ii) (d) —> (i)
Lactic acid is present in curd, acetic acid is present in vinegar, citric acid is present in lemon and oxalic acid is present in tomato.
2.What will be the action of the following substances on litmus paper?
Dry.HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
Ans.Dry HC1 gas will not have any effect on litmus paper. Moistened NH3 gas will turn red litmus blue. Curd, lemon juice, carbonated soft drink will turn blue litmus red. Soap solution will turn red litmus blue.
3.Name the acid present in ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
Ans. Ant sting contains methanoic acid (formic acid). Its chemical formula is HCOOH. The common method to get relief is to apply paste of
4.What happens when nitric acid is added to egg-shell?
Ans. Egg-shell is made up of calcium carbonate which will react with HNO3 to form
5.A student prepared solutions of (i) an acid and (ii) a base in two separate
beakers. She forgot to label the solutions and litmus paper is not available in the laboratory. Since, both the solutions are colourless, how will she distinguish between the two?
Ans.Add phenolphthalein to a portion of each solution in separate test tube. If it turns pink, the beaker contains base whereas if it remains colourless, it is an acid.
If phenolphthalein is not available, pH paper can be used. Acid will turn pH paper red, base will turn pH paper blue.
6.How would you distinguish between baking powder and washing soda by heating?
Ans.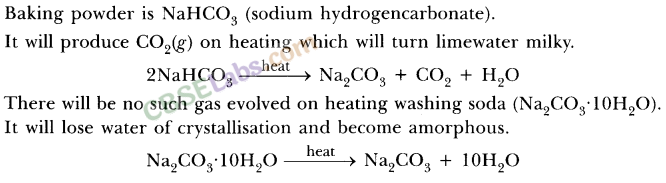
7.Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C.
Ans.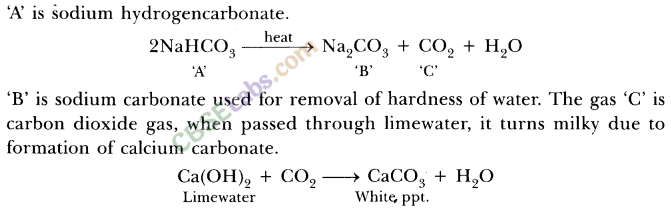
8.In one of the industrial processes used for manufacture of sodium hydroxide, a gas X is formed as by-product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Ans.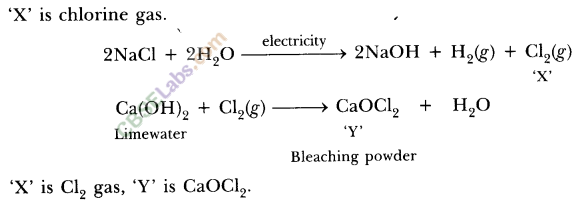
9.What are strong and weak acids? In the following list of acids, separate strong acids from weak acids.
Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Ans. Strong acids are those acids which are completely ionised in aqueous solution. Weak acids are those which do not ionise completely in aqueous solution. Strong adds: HC1,
1O.A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at anode during electrolysis of brine is passed on dry Y, it gives a compound Z, used for disinfecting drinking water. Identify X, Y, G and Z.
Ans.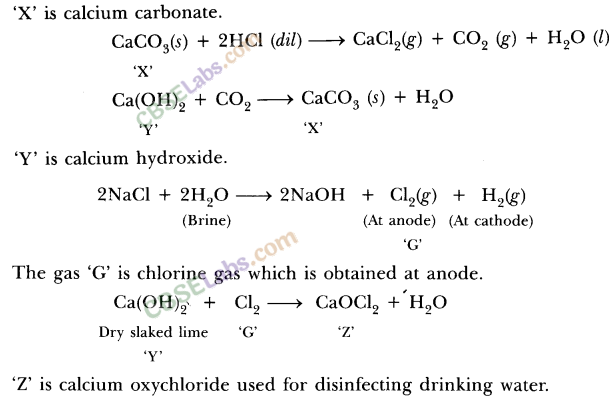
11.A dry pellet of a common base B, when kept in open absorbs moisture and turns sticky. The compound is also a by-product of chlor-alkali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide? Write a balanced chemical equation for one such solution.
Ans.‘B’ is NaOH. It absorbs moisture from atmosphere because it is hygroscopic in nature. It is obtained by product of chlor-alkali process.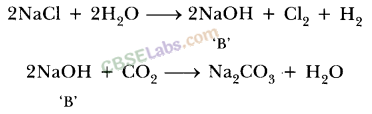
‘B’ reacts with acidic oxide
12.A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in open for sometime, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt. Why does it show such a behaviour? Give the reaction involved.
Ans. Salt is CaS04
moulded into different shapes, as 2 moles of CaS04 share 1 mole of HgO molecule.
When it is left in open, it becomes solid mass CaS042H20 (Gypsum) which cannot be used for moulding purposes as it is hard solid mass.
13.Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.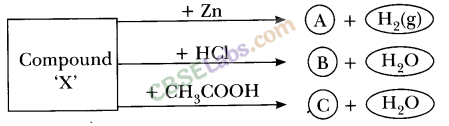
Ans.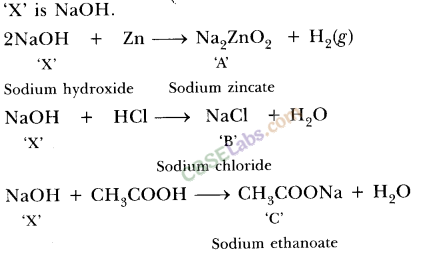
14.Match the important chemicals given in Column (A) with the chemical formulae given in Column (B).
Ans.
15.Fill in the missing data in the following data.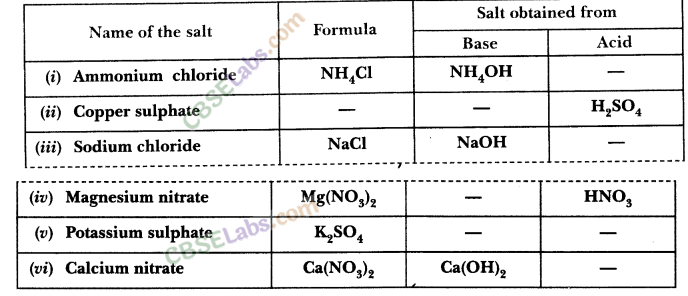
Ans.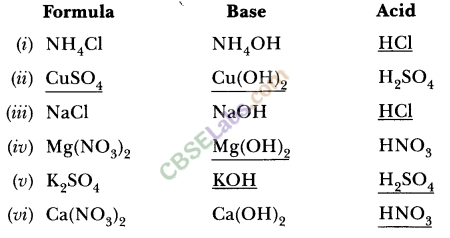
16.When Zinc metal is treated with dilute solution of strong acid, a gas is involved which is utilized in hydrogenation of oil. Name the gas involved. Write the chemical equation for the reaction involved and also write a test to detect the gas formed.
Hydrogen gas is liberated
Ans.
Test: Bring a burning splinter near the gas. The gas will burn with ‘Pop’ sound which shows gas is H2.
17.In the following schematic diagram for the preparation of hydrogen gas as shown in the given figure, what would happen if following changes are made ?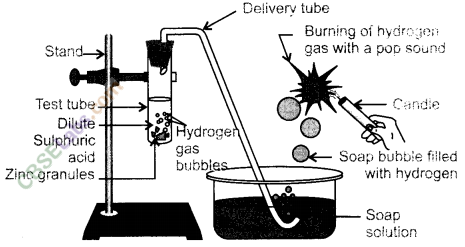
(а)In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b)Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c)In place of zinc, copper turnings are taken.
(d)Sodium hydroxide is taken in place of dilute sulphuric acid and the tube
is heated.
Ans.(a)The reaction will become faster because zinc dust has more surface area.
(b)Nearly same amount of hydrogen gas will be evolved.
(c)No reaction will take place as copper is less reactive than hydrogen.
(d)The reaction will take place and hydrogen gas will be evolved.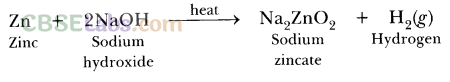
18.For making cake, baking powder is taken. If at home your mother uses baking soda instead of baking powder in cake.
(a)How will it affect the taste of the cake and why?
(b)How can baking soda be converted into baking powder?
(c)What is role of tartaric acid added to baking soda?
Ans.(a)The cake will taste bitter due to formation of sodium carbonate.
(b)Tartaric acid should be added to baking soda to convert it into baking powder.
(c)Tartaric acid neutralizes sodium carbonate formed and will not make the taste bitter.
NCERT Solutions for Class 10 Science All Subject NCERT Solutions
- Chapter 1 Chemical Reactions and Equations
- Chapter 2 Acids, Bases and Salts
- Chapter 3 Metals and Non-metals
- Chapter 4 Carbon and Its Compounds
- Chapter 5 Periodic Classification of Elements
- Chapter 6 Life Processes
- Chapter 7 Control and Coordination
- Chapter 8 How do Organisms Reproduce?
- Chapter 9 Heredity and Evolution
- Chapter 10 Light Reflection and Refraction
- Chapter 11 Human Eye and Colourful World
- Chapter 12 Electricity
- Chapter 13 Magnetic Effects of Electric Current
- Chapter 14 Sources of Energy
- Chapter 15 Our Environment
- Chapter 16 Management of Natural Resources
.png)
.png)