Class 10 Science Chapter 4 Carbon and its Compounds NCERT Solutions
Before getting into the details of NCERT Solutions for Class 10 Science Chapter 4 Carbon And Its Compounds, let’s have an overview of a list of units and sub-units under NCERT Solutions for Class 10 Science Carbon And Its Compounds
- Carbon And Its Compounds
- Bonding In Carbon – The Covalent Bond
- Chemical Properties Of Carbon Compounds
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- Soaps And Detergents
Free download NCERT Solutions for Class 10 Science Chapter 4 Carbon And Its Compounds PDF in Hindi Medium as well as in English Medium for CBSE, Uttarakhand, Bihar, MP Board, Gujarat Board, and UP Board students, who are using NCERT Books based on updated CBSE Syllabus for the session 2019-20.
- कार्बन और इसके यौगिक कक्षा 10 विज्ञान हिंदी में
- Class 10 Carbon and its Compounds Important Questions
- Carbon and its Compounds Class 10 Notes
- Carbon and its Compounds NCERT Exemplar Solutions
- Carbon and Its Compounds Class 10 Extra Questions
- Class 10 Carbon and its Compounds Mind Map
NCERT Solutions for Class 10 Science Chapter 4 Intext Questions
Page Number: 61
Question 1
What would be the electron dot structure of carbon dioxide which has the formula CO2 ?
Answer: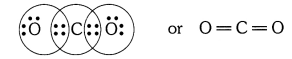
Question 2
What would be electron dot structure of sulphur which is made up of eight atoms of sulphur.
Answer: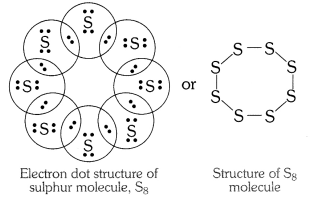
Page Number: 68 – 69
Question 1
How many structural isomers can you draw for pentane ?
Answer:
Three, these are n-pentane, iso-pentane and neo-pentane.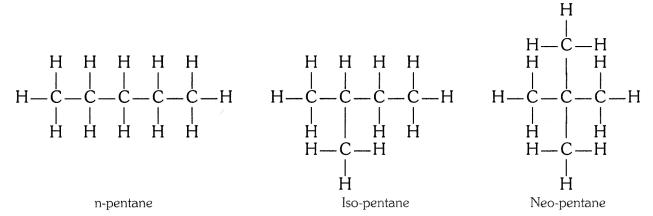
Question 2
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us ?
Answer:
(i) Tetravalency
(ii) Catenation.
Question 3
What will be the formula and electron dot structure of cyclopentane ?
Answer:
The molecular formula of cyclopentane is C5 H10 .
The electron dot structure of cyclopentane is given on the next page.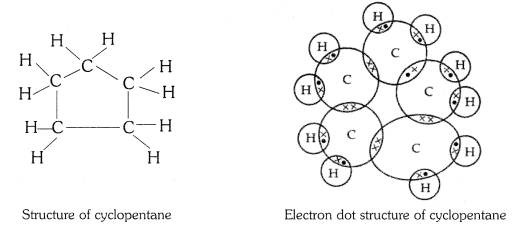
Question 4
Draw the structures for the following compounds :
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) Hexanal
Answer:
(i) Ethanoic acid (CH3COOH)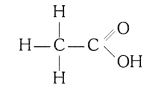
(ii) Bromopentane (C5H11Br)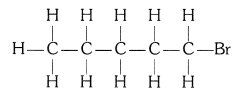
(iii) Butanone (CH3 — CH2 — COCH3)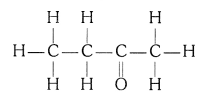
(iv) Hexanal (C5H11CHO)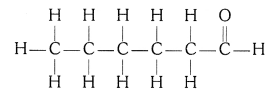
Structural isomers for bromopentane: There are three structural isomers for bromopentane depending on the position of Br at carbon 1, 2, 3.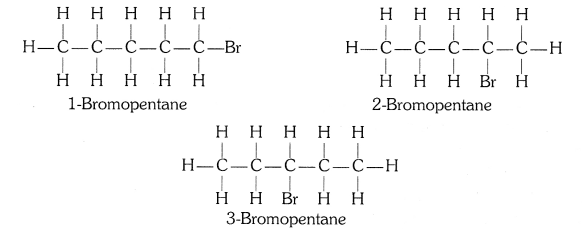
Positions 4 and 5 are same as 1, 2.
Question 5
How would you name the following compounds ?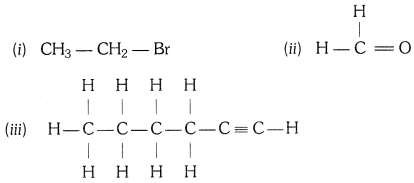
Answer:
(i) Bromoethane
(ii) Methanal
(iii) 1 – Hexyne
Page Number: 71
Question 1
Why is the conversion of ethanol to ethanoic acid an oxidation reaction ?
Answer:
Conversion of ethanol into ethanoic acid is an oxidation reaction because addition of oxygen to a substance is called oxidation. Here, oxygen is added to ethanol by oxidising agent like alkaline potassium permanganate or acidified potassium dichromate and it is converted into acid.
Question 2
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used ?
Answer:
A mixture of ethyne and air is not used for welding because burning of ethyne in air produces a sooty flame due to incomplete combustion, which is not enough to melt metals for welding.
Page Number: 74
Question 1
How would you distinguish experimentally between an alcohol and a carboxylic acid ?
Answer:
Differences between alcohol and carboxylic acid
| Test | Alcohol | Carboxylic acid |
| (i) Litmus test | No change in colour. | Blue litmus solution turns red. |
| (ii) Sodium hydrogen carbonate test | C2H5OH + NaHCO3 → No reaction No brisk effervescence. | CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 Brisk effervescence due to evolution of CO2. |
| (iii) Alkaline potassium permanganate | On heating, pink colour disappears. | Does not happen so. |
Question 2
What are oxidising agents ?
Answer:
Oxidising agents are the substances which give oxygen to another substances or which remove hydrogen from a substance.
For example, acidic K2Cr2O7 is an oxidising agent, that converts (oxidises) ethanol into ethanoic acid.
Page Number: 76
Question 1
Would you be able to check if water is hard by using a detergent ?
Answer:
No, because detergents can lather well even in hard water. They do not form insoluble calcium or magnesium salts (scum). On reacting with the calcium ions and magnesium ions present in the hard water.
Question 2
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes ?
Answer:
It is necessary to agitate to get clean clothes because the soap micelles which entrap oily or greasy particles on the surface of dirty cloth have to be removed from its surface. When the cloth wetted in soap solution is agitated or beaten, the micelles containing oily or greasy dirt get removed from the surface of dirty cloth and go into water and the dirty cloth gets cleaned.
NCERT Solutions for Class 10 Science Chapter 4 Textbook Chapter End Questions
Question 1
Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer:
(b) 7 covalent bonds.
Question 2
Butanone is a four-carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c) Ketone.
Question 3
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer:
(b) The fuel is not burning completely.
Question 4
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
Covalent bond is formed by sharing of electrons so that the combining atoms complete their outermost shell.
In CH3Cl : C = 6, H = 1 and Cl = 17 And their electronic configuration is C – 2,4, H – 1 and Cl – 2, 8, 7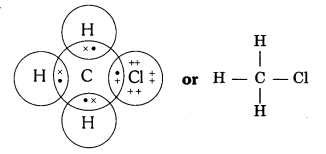
Three hydrogen atoms complete their shells by sharing three electrons (one electron each) of carbon atom.
Chlorine completes its outer shell by sharing its one out of seven electrons with one electron of carbon atom.
Thus carbon atom shares all its four electrons with three hydrogen atoms and one of chlorine atom and completes its outermost shell and single covalent bonds are formed in CH3Cl.
Question 5
Draw the electron dot structures for
(a) ethanoic acid
(b) propanone
(c) H2S
(d) F2.
Answer: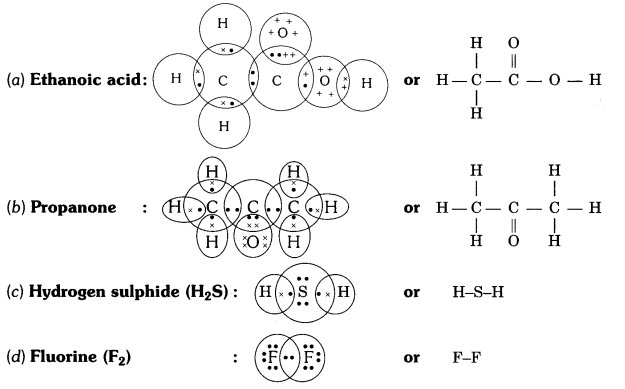
Question 6
What is a homologous series ? Explain with an example.
Answer:
Homologous series : A homologous series is a group of organic compounds having
similar structures and similar chemical properties in which the successive compounds differ by -CH2 group.
Characteristics of homologous series :
(i) All members of a homologous series can be represented by the same general formula. For example, the general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes number of carbon and hydrogen atoms in one molecule of alkane.
(ii) Any two adjacent homologues differ by one carbon atom and two hydrogen atoms in their molecular formulae.
(iii) The difference in the molecular masses of any two adjacent homologues is 14u.
(iv) All the compounds of a homologous series show similar chemical properties.
(v) The members of a homologous series show a gradual change in their physical properties with increase in molecular mass.
For example, general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes number of carbon atoms in one molecule of alkane. Following are the first five members of the homologous series of alkanes (general formula CnH2n+2).
| Value of n | Molecular formula | Name of compound |
| 1 | CH4 | Methane |
| 2 | C2H6 | Ethane |
| 3 | C3H8 | Propane |
| 4 | C4H10 | Butane |
| 5 | C5H12 | Pentane |
Question 7
How can ethanol and ethanoic acid he differentiated on the basis of their physical and chemical properties ?
Answer:
Difference on the basis of physical properties
| Property | Ethanol | Ethanoic acid |
| (i) State | Liquid | Liquid |
| (ii) Odour | Sweet smell | Pungent vinegar-like smell |
| (iii) Melting point | 156 K | 290 K |
| (iv) Boiling point | 351 K | 391 K |
Difference on the basis of chemical properties
| Test | Ethanol | Ethanoic acid |
| (i) Litmus test | No change in the colour of litmus solution. | Blue litmus solution turns red. |
| (ii) Sodium hydrogen carbonate test | C2H5OH + NaHCO3 → No reaction No brisk effervescence. | CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 Brisk effervescence due to evolution of CO2. |
| (iii) Alkaline potassium permanganate | On heating, pink colour disappears. | Does not happen so. |
Question 8
Why does micelle formation take place when soap is added to water ? Will a micell be formed in other solvents such as ethanol also ?
Answer:
Micelle formation takes place when soap is added to water because the hydrocarbon chains of soap molecules are hydrophobic (water repelling) which are insoluble in water, but the ionic ends of soap molecules are hydrophilic (water attracting) and hence soluble in water.
Such micelle formation will not be possible in other solvents like ethanol in which sodium salt of fatty acids do not dissolve.
Question 9
Why are carbon and its compounds used as fuels for most applications ?
Answer:
Carbon and its compounds give a large amount of heat per unit weight and are therefore, used as fuels for most applications.
Question 10
Explain the formation of scum when hard water is treated with soap.
Answer:
Hard water contains salts of calcium and magnesium. Calcium and magnesium on reacting with soap form insoluble precipitate called scum. The scum formation lessens the cleansing property of soaps in hard water.
Question 11
What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
Red litmus will turn blue because soap is alkaline in nature. Blue litmus remains blue in soap solution.
Question 12
What is hydrogenation ? What is its industrial application ?
Answer:
The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydro-carbon is called hydrogenation. The process of hydrogenation takes place in the presence of nickel (Ni) or palladium (Pd) metals as catalyst.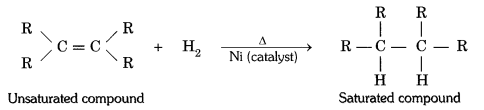
Application : The process of hydrogenation has an important industrial application. It is used to prepare vegetable ghee (or vanaspati ghee) from vegetable oils.
Question 13
Which of the following hydrocarbons undergo addition reactions :
C2H6, C3H8, C3H6, C2H2 and CH4
Answer:
Addition reactions take place only in unsaturated hydrocarbons. So addition reaction take place only in C3H6 and C2H2.
Question 14
Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer:
Butter is a saturated carbon compound while cooking oil is an unsaturated carbon compound. An unsaturated compound decolourises bromine water, while a saturated compound cannot decolourise it. So we can distinguish chemically between a cooking oil and butter by the bromine water. Add bromine water to a little of cooking oil and butter taken in separate test-tubes.
- Cooking oil decolourises bromine water showing that it is an unsaturated compound.
- Butter does not decolourise bromine water showing that it is a saturated compound.
Question 15
Explain the mechanism of the cleaning action of soaps.
OR
Explain the cleansing action of soaps. [CBSE 2015 (Delhi)]
Answer:
When a dirty cloth is put in water containing dissolved soap, then the hydrocarbon end of the soap molecules in micelle attach to the oil or grease particles present on the surface of dirty cloth. In this way the soap micelle entraps the oily or greasy particles by using its hydrocarbon ends. The ionic ends of the soap molecules in the micelles, however, remain attached to water. When the dirty cloth is agitated in soap solution, the oily and greasy particles present on its surface and entrapped by soap micelles get dispersed in water due to which the soap water becomes dirty but the cloth gets cleaned. The cloth is cleaned thoroughly by rinsing in clean water a number of times.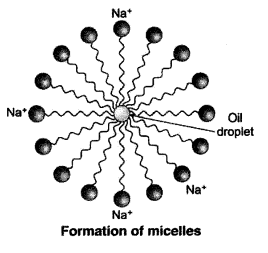
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 10 |
| Subject | Science |
| Chapter | Chapter 4 |
| Chapter Name | Carbon and its Compounds |
| Number of Questions Solved | 28 |
| Category | NCERT Solutions |
NCERT Solutions for Class 10 Science Chapter 4 Carbon and its Compounds
Carbon compounds: Covalent bonding in carbon compounds, Versatile nature of carbon, Homologous series, Nomenclature of carbon compounds containing functional groups, (halogens, alcohol, ketones, aldehydes, alkanes, and alkynes), difference between saturated hydrocarbons and unsaturated hydrocarbons. Chemical properties of carbon compounds (combustion, oxidation, addition and substitution reaction). Ethanol (only properties and uses), Ethanoic acid (only properties and uses), soaps and detergents.
Formulae Handbook for Class 10 Maths and Science
Question 1
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Solution: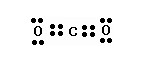
Question 2
What would be the electron dot structure of a molecule of sulphur, which is made up of eight atoms of sulphur? (Hint – The eight atoms of sulphur are joined together in the form of a ring.)
Solution: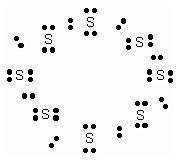
Question 3
How many structural isomers can you draw for pentane?
Solution:
We can draw 3 structural isomers for pentane.
Question 4
What are the two properties of carbon that lead to the huge number of carbon compounds we see around us?
Solution:
Due to its large valency, carbon atoms can form covalent bonds with a number of carbon atoms as well as with a large number of other atoms such as hydrogen, oxygen, nitrogen, sulphur, chlorine and many more atoms. This leads to the formation of a large number of organic compounds.
Question 5
What will be the formula and electron dot structure of Cyclopentane?
Solution: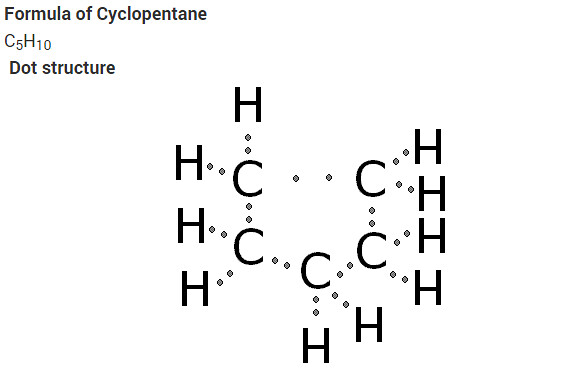
Question 6
Draw the structures for the following compounds.
i. Ethanoic acid
ii. Bromopentane
iii. Butanone
iv. Hexanal
Solution: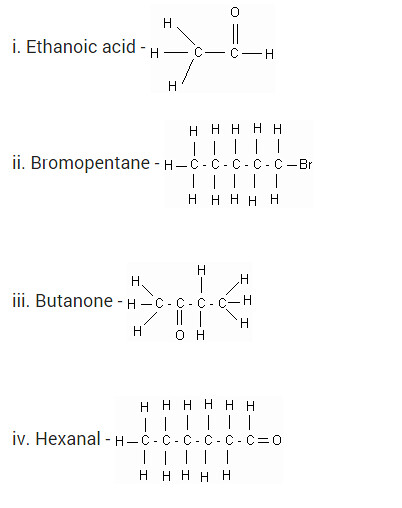
Question 7
How would you name the following compounds?
Solution:
i. Ethyl bromide
ii. Formaldehyde
iii. Hexyne
Question 8
Why is the conversion of ethanol to Ethanoic acid an oxidation reaction?
Solution:
The conversion of ethanol into ethanoic acid is called an oxidation reaction because oxygen is added to it during this conversion.
Question 9
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Solution:
When a mixture of oxygen and ethyne is burnt, it burns completely producing a blue flame. This blue flame is extremely hot which produced a very high temperature which is used for welding metals. But the mixture of ethyne and air is not used for welding purposes because burning of ethyne in air produces a sooty flame, which is not enough to melt metals for welding.
Question 10
What are oxidizing agents?
Solution:
Oxidizing agents are the substances that gain electrons in an redox reaction and whose oxidation number is reduced.
Question 11
Explain the nature of the covalent bond using the bond formation of CH3Cl.
Solution:
CH3Cl(methyl chloride) is made up of one carbon atom, three hydrogen atoms and one chlorine atom. Carbon atom has 4 valence electrons, each hydrogen atom has one valence electron, and a chlorine atom has 7 valence electrons. Carbon atom shares its four valence electrons with three hydrogen atoms and 1 chlorine atom to form methyl chloride as follows:
From the above reaction, in the dot structure of methyl chloride (CH3Cl) there are four pairs of shared electrons between carbon and other atoms. Each pair of shared electrons constitutes one single covalent bond. So, methyl chloride has four single covalent bonds.
Question 12
Draw the electron dot structures for-
Solution: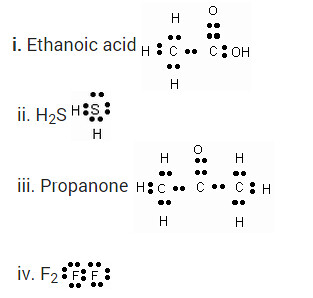
Question 13
What is a homologous series? Explain with an example.
Solution:
Homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and shows a gradation in physical properties as a result of increase in molecular size and mass. For example, methane has a lower boiling point than ethane since it has more intermolecular forces with neighbouring molecules. This is because of the increase in the number of atoms making up the molecule.
Question 14
How can ethanol and Ethanoic acid be differentiated on the basis of their physical and chemical properties?
Solution:
(i) Ethanol has a pleasant smell whereas ethanoic acid has the smell of vinegar.
(ii) Ethanol has a burning taste whereas ethanoic acid has a sour taste.
(iii) Ethanol has no action on litmus paper whereas ethanoic acid turns blue litmus paper red.
(iv) Ethanol has no reaction with sodium hydrogencarbonate but Ethanoic acid gives brisk effervescence with sodium hydrogencarbonate.
Question 15
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Solution:
Micelle formation takes place when soap is added to water. This is because when soap is added to water in which dirty clothes are soaked, the two parts of the soap molecule dissolves in two different mediums. The organic tail dissolves in the dirt, grime or grease and the ionic head dissolves in water. When the clothes are rinsed or agitated, the dirt gets pulled out of the clothes in the water by the soap molecule. In this way the soap does its cleaning work on dirty and grimy clothes or hands.
The soap molecules actually form a closed structure because of mutual repulsion of the positively charged heads. This structure is called a micelle.
Question 16
Why are carbon and its compounds used as fuels for most applications?
Solution:
Carbon and its compounds are used as fuels for most of the applications because they burn in air releasing a lot of heat energy.
Question 17
Explain the formation of scum when hard water is treated with soap.
Solution:
The precipitate form of scum is formed when soap is used for washing clothes. With hard water, a large amount of soap is wasted in reacting with the calcium and magnesium ions of hard water to form an insoluble precipitate. The precipitate form formed by the action of hard water on soap, sticks to the clothes being washed and interferes with the cleaning ability of the additional soap. This makes the cleaning of clothes difficult.
Question 18
What change will you observe if you test soap with litmus paper (red and blue)?
Solution:
Soap is the salt of a strong base (NaOH) and a weak acid (carboxylic acid), so a solution of soap in water is basic in nature. Being basic, a soap solution turns red litmus paper blue.
Question 19
What is hydrogenation? What is its industrial application?
Solution:
It is a class of chemical reactions in which the net result is addition of hydrogen (H2) to unsaturated organic compounds such as alkenes, alkynes, etc. Hydrogenation is widely applied to the processing of vegetable oils and fats. Complete hydrogenation converts unsaturated fatty acids to saturated ones.
Question 20
C2H5, C3H8, C3H6, C2H2 and CH4
Solution:
Alkenes and alkynes (unsaturated hydrocarbons) undergo addition reactions. From the above hydrocarbons C2H2 is an alkyne, whereas C3H6 is an alkene. So, C3H6 and C2H2 will undergo addition reactions.
Question 21
Give a test that can be used to differentiate chemically between butter and cooking oil.
Solution:
Bromine water test can be used to differentiate chemically between butter and cooking oil. Add bromine water to a little of cooking oil and butter taken in separate test tubes. <font
a. Decolourising of bromine water by cooking oil (unsaturated compound)
b. Butter (saturated compound) does not decolourise bromine water
Question 22
Explain the mechanism of the cleaning action of soaps.
Solution:
We all know that soap is used to remove dirt and and grime from substances. Generally dirt and grime get stuck because they have an oily component, which is difficult to remove, by plain brushing or washing by water. A soap molecule has two parts, a head and a tail i.e. the long chain organic part and the functional group –COO– Na+.
A soap molecule has a tadpole like structure shown below.
The organic part is water insoluble but is soluble in organic solvents or in oil or grease. The ionic part is soluble in water, as water is a polar solvent. When soap is added to water in which dirty clothes are soaked, the two parts of the soap molecule dissolve in two different mediums. The organic tail dissolves in the dirt, grime or grease and the ionic head dissolves in water. When the clothes are rinsed or agitated, the dirt gets pulled out of the clothes, by the soap molecule. In this way soap does its cleaning work on dirty and grimy clothes or hands.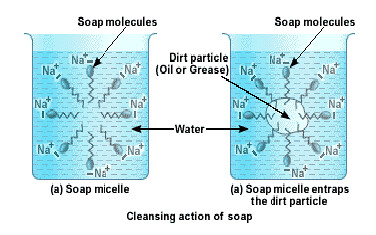
The soap molecules actually form a closed structure because of mutual repulsion of the positively charged heads. This structure is called a micelle. The micelle pulls out the dirt and grime more efficiently.
Question 23
Would you be able to check if water is hard by using a detergent?
Solution:
We would not be able to check whether a sample of water is hard by using a detergent, this is because a detergent forms lather easily even with hard water.
Question 24
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat ii with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Solution:
It is necessary to shake to get clean clothes because the soap micelles, which entrap oily or greasy particles on the surface of dirty clothes, have to be removed from their surface. When the clothes which are wet by soap solution are beaten, the micelles containing oil or greasy dirt particles get removed from the surface of dirty clothes and go into water and the dirty cloth gets cleaned.
Multiple Choice Questions (MCQs) [1 Mark each]
Question 1.
Buckminster fullerene is an allotropic form of [NCERT Exemplar]
(a) phosphorus
(b) sulphur
(c) carbon
(d) tin
Answer:
(c) Buckminster fullerene is an allotrope of carbon containing clusters of 60 carbon atoms joined together to form spherical molecules. Its formula isC60 (C-sixty). It is a dark solid at room temperature and as compared to another allotropic form of carbon (diamond and graphite), it is neither very hard nor soft.
Question 2.
The hetero atoms present in
CH3 – CH2 – O – CH2 – CH2Cl are [NCERT Exemplar]
(i) oxygen
(ii) carbon
(iii) hydrogen
(iv) chlorine
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer:
(d) Atoms other than C and H, if present in organic compound, are called heteroatoms.
Question 3.
In which of the following .compounds -OH is the functional group? [NCERT Exemplar]
(a) Butanone
(b) Butanol
(c) Butanoic
(d) Butanal
Answer:
(b) Butanol, CH3—CH2—CH2—CH2—OH
The general formula of alcohols is CnH2n+1— OH.
For butanol, n = 4. So, formula is
C4H9—OH or CH3—CH2—CH2—CH2—OH
Question 4.
The soap molecule has a [NCERT Exemplar]
(a) hydrophilic head and a hydrophobic tail
(b) hydrophobic head and a hydrophilic tail
(c) hydrophobic head and a hydrophobic tail
(d) hydrophilic head and a hydrophilic tail
Answer:
(a) A soap molecule is made up of two parts- a long hydrocarbon part and a short ionic part —COONa+ group. The long hydrocarbon chain is hydrophobic (water repelling) and ionic portion is hydrophilic (water attracting).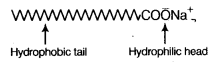
Question 5.
Structural formula of benzene is [NCERT Exemplar]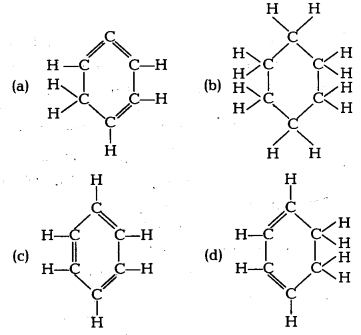
Answer:
(c) Benzene molecule contains alternate single and . double bonds. Its formula is C6H6. In structure (b) formula is C6H12. In structure (a) double bond is not at alternate position. In (d) formula is C6H8.
Question 6.
Which of the following is not a straight chain hydrocarbon? [NCERT Exemplar]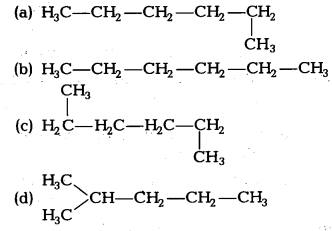
Answer: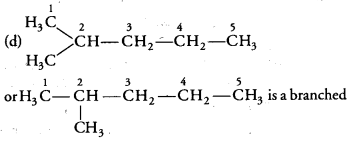
chain hydrocarbon not straight chain hydrocarbon. Rest three are straight chain hydrocarbons.
Question 7.
Which among the following are unsaturated hydrocarbons? [NCERT Exemplar]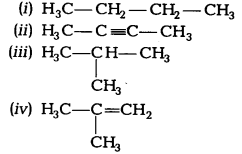
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer:
(c) Unsaturated hydrocarbons have double or triple bond in the structure. Both (ii) and (iv) structures have triple and double carbon-carbon bonds respectively.
Question 8.
Chlorine reacts with saturated hydrocarbons at room temperature in the [NCERT Exemplar]
(a) absence of sunlight
(b) presence of sunlight
(c) presence of water
(d) presence of hydrochloric acid
Answer:
(b) Chlorine reacts with saturated hydrocarbon at room temperature in the presence of sunlight.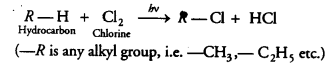
Question 9.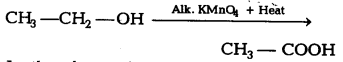
In the above given reaction, alk.KMnO4 acts as [NCERT Exemplar]
(a) reducing agent
(b) oxidising agent
(c) catalyst agent
(d) dehydrating
Answer:
(b) KMnO4 acts as oxidising agent, because it removes hydrogen from CH3CH2OH and adds one oxygen to it.
Question 10.
Butanone is a four carbon compound with functional group [NCERT Exemplar]
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c) In butanone, the functional group is![]()
Question 11.
Identify the unsaturated compounds from the following [NCERT Exemplar]
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (ii) and (iii)
Answer:
(d) Propene, CH3CH=CH2 (ii) and propyne, CH3— C = CH (iii) both have double and triple bonds, respectively, hence are unsaturated compounds.
Question 12.
Which of the following does not belong to the same homologous series? [NCERT Exemplar]
(a) CH4
(b) C2H6
(c) C3H8
(d) C4H8
Answer:
(d) Because succesive members of a homologous series differ by —CH2 unit.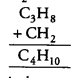
Thus, C4H10 is the next member of this series. So, homologous series of alkanes is:
methane (CH4), ethane (C2H6), propane (C3H8) and butane (C4H10).
So, C4H8 does not belong to the homologous series.
Question 13.
Ethane with molecular formula C2H6 has [NCERT Exemplar]
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer:
(b) Structure formula of ethane (C2H6) is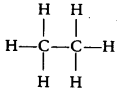
It is clear that it has 7 covalent bonds.
Question 14.
Which of the following are correct structural isomers of butane? [NCERT Exemplar]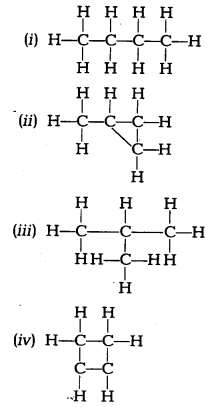
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:
(a) Structure (i) is n-butane and structure (iii) is iso-butane. Since, molecular formula is same, only structures are different. So, (i) and (iii) are isomers while structures (ii) and (iv) have molecular formulaC4H8.
Question 15.
In the soap micelles, [NCERT Exemplar]
(a) the ionic end of soap is on the surface of the cluster while the carbon chain is in the interior of the cluster
(b) ionic end of soap is in the interior of the cluster and the carbon chain is out of the cluster
(c) Both ionic end and carbon chain are in the interior of the cluster
(d) Both ionic end and carbon chain are on the exterior of the cluster
Answer:
(a) A ‘spherical aggregate of soap molecules’ in the soap solution in water is called a ‘micelle’. In a soap micelle, the soap molecules are arranged readily with hydrocarbon ends directed towards the centre and ionic ends directed outwards.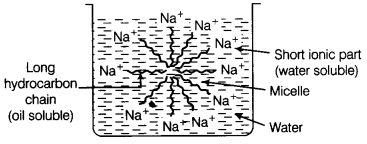
Question 16.
Vinegar is a solution of [NCERT Exemplar]
(a) 50% – 60% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 50% – 60% acetic acid in water
Answer:
(c) A 5%-8% solution of acetic acid in water is called vinegar.
Question 17.
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of [NCERT Exemplar]
(a) addition reaction
(b) substitution reaction
(c) displacement reaction
(d) oxidation reaction
Answer:
(a) Oils are unsaturated compounds containing double bonds. Addition reactions are characteristic property of unsaturated hydrocarbons. The given reaction is an example of addition reaction.
Question 18.
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of [NCERT Exemplar]
(a) helium
(b) neon
(c) argon
(d) krypton
Answer:
(b) Electronic configuration of carbon (C) = 2, 4 when it forms four covalent bonds by sharing its four valence electrons with hydrogen, it forms CH4 molecule like this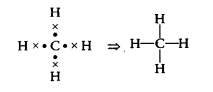
Now, electronic configuration of C in CH
4 = 2, 8.
Atomic number of Ne is 10. Its electronic K L configuration is 2,8. Therefore, after the formation of four bonds, carbon attains the electronic configuration of neon.
Question 19.
Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised.
(ii) carboxylic acids are completely ionised.
(iii) mineral acids are partially ionised.
(iv) carboxylic acids are partially ionised.
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:
(a) Mineral acids are strong acids which ionise almost completely and carboxylic acids are weak acids which ionise only pardally.
Question 20.
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that [NCERT Exemplar]
(a) food is not cooked completely
(b) the fuel is not burning completely
(c) fuel is wet
(d) fuel is burning completely
Answer:
(b) The unburnt particles of the fuel present in smoke blacken the vessel from outside.
Question 21.
The reaction in which a reagent (partially or completely) replaces atom or group of atoms from saturated compounds or A are called B reaction.
Here, A and B respectively refers to
(a) unsaturated compounds, addition
(b) unsaturated compounds, substitution
(c) benzene, substitution
(d) alkene, addition
Answer:
(c) Substitution reaction is usually given by saturated compounds and benzene. Unsaturated compounds usually give addition reactions.
Question 22.
The table gives information about some esters and the fragrance they produce.
| Ester | Fragrance |
| Ethyl methanoate | Rum |
| Methyl butanoate | Apple |
| Ethyl butanoate | Pineapple |
| Propyl ethanoate | Pear |
Which structure do the ester compounds in the table have in common?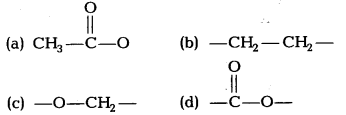
Answer:
(d) All esters have the common structure of carboxylic group represented by the suffix date.
NCERT Solutions for Class 10 Science Chapter 4 Carbon and Its Compounds (Hindi Medium)















Class 10 Science Carbon and its Compounds Mind Map
Carbon is an important constituent of the foods, fuels, household and commercial articles, textile fabrics, perfumes, explosives, dyes, war gases etc. The Earth’s crust has only 0.02% carbon in the form of minerals (like petroleum, coal, carbonates and hydrogen carbonates) and the atmosphere comprises of only 0.03% of carbon dioxide
Allotropes of Carbon
An element, in different forms, having different physical properties but similar chemical properties is known as allotropes of that element.
Carbon has three well known allotropes which are graphite, diamond and buck minster fullerene.
Graphite:
- In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array.
- One of these bonds is a double-bond, and thus the valency of carbon is satisfied.
- Graphite structure is formed by the hexagonal arrays being placed in layers one above the other.
- Unlike other non-metals graphite is a very good conductor of electricity.
Diamond:
- In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
- Diamond is the hardest substance known while graphite is smooth and slippery.
- Diamonds can be synthesized by subjecting pure carbon to very high pressure and temperature.
- These synthetic diamonds are small but are otherwise indistinguishable from natural diamonds.
Fullerene:
This is another class of carbon allotropes. The first one to be identified was C-60 which has carbon atoms arranged in the shape of a football. Since this looked like the geodesic dome designed by the US architect Buckminster Fuller, the molecule was named fullerene.
Saturated and Unsaturated Hydrocarbons
Saturated hydrocarbons: Compounds in which carbon- carbon atoms & carbon-hydrogen atoms are held together by single bonds. General formula is CnH2n+2.
Unsaturated hydrocarbons: Compounds consist of a single, double or triple bond between carbon-carbon atoms.
These double-bonded compounds are called alkenes & the triple-bonded compounds are called alkynes.
The general formula for alkenes is CnH2n & for alkynes the same is CnH2n-2.
Cycloalkanes: These hydrocarbons possess one or multiple carbon rings. The hydrogen atom is attached to the carbon ring.
Aromatic hydrocarbons: These are also called as arenes. Arenes are compounds which consist of at least one aromatic ring.
Bonding in Carbon
- Atomic number of carbon = 6
- Electronic configuration has 2 electrons in KL shell and 4 electrons in L shell.
- Carbon shares its four valence electrons with other atoms of carbon or with atoms of other elements. These electrons contributed by the atoms, for mutual sharing in order to acquire the stable noble gas configuration is called covalencv of that atom.
- Hence, carbon shows tetracovalency.
- The simplest molecule formed by sharing of electrons (i.e., covalent bonds), can be represented by electron dot structure.
Versatile Nature of Carbon
- Catenation: It is the unique property of self linkage of carbon atoms by means of covalent bonds to form straight chains, or branched chains, or the rings of different sizes.
- Tetracovalency: Due to small size, and presence of four valence electrons, carbon can fonn strong bonds with other carbon atoms, hydrogen, oxygen, nitrogen, or sulphur, etc. For example, compounds of carbon with hydrogen are called hydrocarbons.
- Multiple Bond Formation: Due to small size, it forms multiple bonds (i.e., double bonds or triple bonds) with other elements as well as with its own atoms.
- Isomerism: The phenomenon by means of which the carbon compounds with same molecular formula show different structures, and properties.
Soaps: Ester of higher fatty acids is called soap. It is manufactured by the reaction of caster of higher fatty acid with sodium hydroxide. The sodium salt so formed has cleansing property.
Detergents: Soap cannot form lather in hard water. To overcome this problem, detergents were introduced. Detergent is also known as soapless soap. Detergent is sodium salt of benzene sulphonic acid or sodium salt of long chain alkyl hydrogen sulphate.
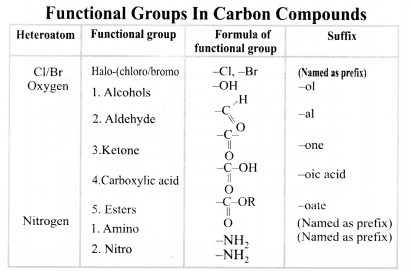
Nomenclature of Carbon Compounds: IUPAC Name
Rales for Nomenclature of Organic Compounds:
- Identify the number of carbon atoms in the compound & name the compound according to the number of carbon atoms.
- If any functional group is present, add a ‘prefix’ or ‘suffix’ specified for the functional group with the name of the parent compound.
- Prefix is added for only Halo group and for rest of the functional groups suffix is added.
- If the name of the functional group is to be given as a suffix, the name of the carbon chain is modified by deleting the final ‘e’ and adding the appropriate suffix.
- In the case of unsaturated hydrocarbon if double bond is present in the carbon chain, then ‘ene’ will be added in the parent name of compound after omitting ‘ane’ and if triple bond is present in the carbon chain, then ‘yne’ will be added.
Properties of Ethanol
- Ethanol exists in liquid state at room temperature.
- Mixture of alcohol to ethane results in the fonnation of ethanol & it is the active ingredient of all alcoholic drinks. Even a small quantity of ethanol if consumed can causes drunkenness.
- Being a good solvent, it is also used in medicines like tincture of iodine, cough syrups, and many other tonics.
- Reaction with sodium –
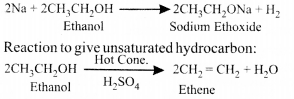
Properties of Ethanoic Acid
- Ethanoic acid commonly known as acetic acid belongs to a group of carboxylic acids.
- Vinegar used in our day to day life is a 5-8% solution of acetic acid in water. It is extensively used as a preservative in pickles.
- Pure ethanoic acid has a melting point of290 K due to which it
often freezes in cold climates giving rise to its name as glacial acetic acid.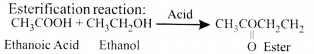
- Reaction with a base: NaOH + CH3COOH → CH3COONa + H2O
- Reaction with carbonates and hydrogen carbonates:
2CH3COOH + NA2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Homologous Series
- A series of carbon compounds in which same functional group substitutes the hydrogen atom is called a homologous series.
- These compounds have similar chemical properties due to the presence of same kind of functional group. For e.g. series of alkanes i.e. methane, ethane, propane, butane and so on is a homologous series.
- Similarly the series of alkene & alkynes also forms homologous series.
- The series like methanol, ethanol, propanol, butanol and so on is also a homologous series. The functional group attached to these compounds is alcohol.
- With the increase in molecular mass in a homologous series the physical properties like melting points, boiling points & solubility in a particular solvent increases.
- But the chemical properties of a homologous series determined by the functional group remain same.
Chemical Properties of Carbon Compounds
Combustion Reactions: When Carbon & its compounds bum in the presence of Oxygen (or air), they give CO2, heat & light.
E.g.: C + O2 → CO2 + Heat + Light.
Oxidation Reactions: Though combustion is generally an oxidation reaction, not all oxidation reactions are combustion reactions. Oxidation is also carried out by using oxidizing agents (Oxidants).
Addition reactions: Unsaturated organic compounds, like alkenes and alkynes, undergo addition reactions to become saturated in nature.
E.g. CH2 = CH2 + H2 + (Nickel catalyst) → CH3– CH3
Substitution Reaction: A Substitution reaction is one in which an atom or a group of atoms (functional group) in the compound are replaced by another atom ( or group of atoms). Substitution reactions are single displacement reactions.
E.g.: CH4 + Cl2 + Sunlight → CH3Cl + HCl
Now that you are provided all the necessary information regarding NCERT Solutions for class 10 Science chapter 4. We hope this detailed article on NCERT Solutions for Class 10 Science Carbon and its compounds is helpful. If you have any query regarding this article or NCERT Solutions for Class 10 Science, leave your comments in the comment section below and we will get back to you as soon possible.
Important Questions of Carbon and its Compounds Class 10 Science Chapter 4
Question 1.
Covalent compounds have low melting and boiling point. Why? (2020)
Answer:
Covalent compounds have low melting and boiling points because the forces of attraction between molecules of covalent compounds are very weak. On applying a small amount of heat these molecular forces break.
Question 2.
What are covalent compounds? Why are they different from ionic compounds? List their three characteristic properties. (Delhi 2016)
Answer:
Covalent compounds are those compounds which are formed by sharing of valence electrons between the atoms e.g., hydrogen molecule is formed by mutual sharing of electrons between two hydrogen atoms.
They are different from ionic compounds as ionic compounds are formed by the complete transfer of electrons from one atom to another e.g., NaCl is formed when one valence electron of sodium gets completely transferred to outer shell of chlorine atom. The characteristic properties of covalent compounds are:
(i) They are generally insoluble or less soluble in water but soluble in organic solvents.
(ii) They have low melting and boiling points.
(iii) They do not conduct electricity as they do not contain ions.
Question 3.
What are covalent bonds? Show their formation with the help of electron dot structure of methane. Why are covalent compounds generally poor conductors of electricity? (Delhi 2013C)
Answer:
Covalent bonds are those bonds which are formed by sharing of the valence electrons between two atoms. Electron dot structure of methane is shown in the figure.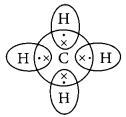
Covalent compounds are generally poor conductors ol electricity because they do not have tree electrons or ions.
Question 4.
Give reasons for the following:
(i) Element carbon forms compounds mainly by covalent bonding.
(ii) Diamond has high melting point.
(iii) Graphite is a good conductor of electricity. (3/5, Foreign 2011)
Answer:
(i) As carbon has four valence electrons and it can neither loose nor gain lour electrons thus, it attains noble gas configuration only by sharing of electrons. I bus, it forms covalent compounds.
(ii) In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. This makes diamond the hardest known substance. Thus, it has high melting point.
(iii) In graphite, each carbon atom is bonded to three other carbon atoms by covalent bonds in the same plane giving a hexagonal array. Thus, only three valence electrons are used for bond formation and hence, the fourth valence electron is free to move. As a result, graphite is a good conductor of electricity.
Question 5.
What is methane? Draw its electron dot structure. Name the type of bonds formed in this compound. Why are such compounds
(i) poor conductors of electricity and
(ii) have low melting and boiling points?
What happens when this compound burns in oxygen? (Delhi 2019)
Answer:
Methane is the first member of alkane series having formula CH4.
Refer to answer 3.
(ii) Refer to answer 1.
When methane is burnt in presence of oxygen then carbon dioxide will be produced.
CH4 + O2 → CO2 + H2O + heat + light
Question 6.
Elements forming ionic compounds attain noble gas electronic configuration by either gaining or losing electrons from their valence shells. Explain giving reason why carbon cannot attain such a configuration in this manner to form its compounds. Name the type of bonds formed in ionic compounds and in the compounds formed by carbon. Also explain with reason why carbon compounds are generally poor conductors of electricity. (Foreign 2015, AI 2014)
Answer:
Ionic compounds are formed either by gaining or losing electrons from the outermost shells, but carbon which has four electrons in its outermost shell cannot form ionic bonds because
1. If carbon forms ionic bonds by gaining four electrons to attain a noble gas configuration then it would be difficult for six protons in the nucleus to hold ten electrons.
2. If carbon forms ionic bonds by loss of four electrons then it would require a lot of energy to remove these electrons from outermost shell.
Due to these reasons carbon forms covalent bonds by sharing the valence electrons.
Type of bonds formed in ionic compounds are called electrovalent bonds and the type of bonds formed in carbon compounds are called covalent bonds.
Refer to answer 3.
Question 7.
State the reason why carbon can neither form C4+ cations nor C4- anions, but forms covalent compounds. Also state reasons to explain why covalent compounds :
(i) are bad conductors of electricity?
(ii) have low melting and boiling points? (Delhi 2014)
Answer:
Refer to answer 6.
(i) Refer to answer 3.
(ii) Refer to answer 1.
Question 8.
Name a cyclic unsaturated carbon compound. (2020)
Answer:![]()
Question 9.
Assertion (A) : Following are the members of a homologous series :
CH3OH, CH3CH2OH, CH3CH2CH2OH
Reason (R) : A series of compounds with same functional group but differing by -CH2 unit is called homologous series.
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true. (2020)
Answer:
(a): The given compounds are members of homologous series of alcohol.
Question 10.
Write the molecular formula of first two members of homologous series having functional group -Cl. (Delhi 2017)
Answer:
The molecular formula of first two members of homologous series having -Cl functional group are CH3Cl and CH3CH2Cl.
Question 11.
Write the molecular formula of first two members of homologous series having functional group -OH. (Delhi 2017)
Answer:
The molecular formula of first two members of homologous series having -OH functional group are CH3OH and CH3CH2OH.
Question 12.
Write the molecular formula of the 2nd and 3rd member of the homologous series whose first member is ethene. (AI 2017)
Answer:
Homologous series of alkenes have general formula, CnH2n whose first member is ethene.
2nd member of homologous series of alkenes is C3H6 i.e., propene.
3rd member of homologous series of alkenes is C4H8 i.e., butene.
Question 13.
Write the molecular formula of the 2nd and 3rd member of the homologous series whose first member is methane. (AI 2017)
Answer:
Methane, CH4 is an alkane. Alkanes have general formula, CnH2n+2.
2nd member of homologous series of alkanes is C2H6 i.e., ethane.
3rd member of homologous series of alkanes is C3H8 i.e., propane.
Question 14.
Write the next homologue of each of the following:
(i) C2H4
(ii) C4H6 (Delhi 2016)
Answer:
(i) C2H4 belongs to alkene series having general formula, CnH2n.
Thus, next homologue will be C3H2×3 = C3H6
(ii) C4H6 belongs to alkyne series having general formula, CnH2n-2.
Thus, next homologue will be C5H2×5-2 = C5H8
Question 15.
Name the following compounds :
(a) CH3 – CH2 – OH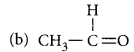
Answer:
(a) CH3 – CH2 – OH : Ethanol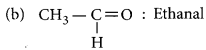
Question 16.
Select saturated hydrocarbons from the following : C3H6; C5H10; C4H10; C6H14; C2H4
Answer:
Saturated hydrocarbons have general formula, CnH2n+2.
Among the given compounds only C4H10 and C6H14 satisfy the above formula. Thus, these are saturated hydrocarbons.
Question 17.
Write the name and structure of an alcohol with three carbon atoms in its molecule. (AI 2016)
Answer:
An alcohol with three carbon atoms in its molecule is propanol. The structure of propanol is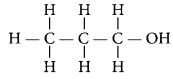
Question 18.
Write the name and structure of an alcohol with four carbon atoms in its molecule. (AI 2016)
Answer:
An alcohol with four carbon atoms is butanol and its structure is :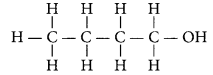
Question 19.
Write the name and structure of an aldehyde with four carbon atoms in its molecule. (AI 2016)
Answer:
An aldehyde with four carbon atoms is butanal and its structure is.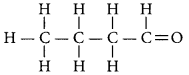
Question 20.
Which element exhibits the property of catenation to maximum extent and why? (Foreign 2016)
Answer:
Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. Carbon shows catenation due to its small size and Stronger carbon-carbon bond strength.
Question 21.
Write the name and molecular formula of the fourth member of alkane series. (Foreign 2016)
Answer:
The general formula of the alkane series is CnH2n+2. For fourth member of alkane series, n = 4
∴ C4H2×4+2 = C4H10 i.e., butane.
Question 22.
What is homologous series of carbon compounds? (Foreign 2016)
Answer:
A homologous series is the family of organic compounds having the same functional group, similar chemical properties but the successive (adjacent) members of the series differ by a -CH2 unit or 14 mass units.
Question 23.
Write the name and formula of the 2nd member of homologous series having general formula CnH2n. (Delhi 2015)
Answer:
Refer to answer 12.
Question 24.
Write the name and formula of the 2nd member of homologous series having general formula CnH2n+2. (Delhi 2015)
Answer:
Refer to answer 13.
Question 25.
Write the name and formula of the 2nd member of homologous series having general formula CnH2n-2. (Delhi 2015)
Answer:
General formula, CnH2n-2 belongs to alkyne series. The second member of this series is propyne i.e., (C3H4) or CH3 – C ≡ CH.
Question 26.
Write the number of covalent bonds in the molecule of ethane. (AI2015, Delhi 2014)
Answer:
The structural formula of ethane (C2H6) is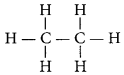
There are total 7 covalent bonds. Six C – H covalent bonds and one C – C covalent bond.
Question 27.
Write the number of covalent bonds in the molecule of butane, C4H10. (AI 2015)
Answer:
Butane (C4H10) has the following structural formula as: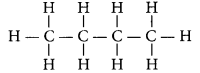
Total number of covalent bonds is 13 in which there are 10 C – H and 3 C – C covalent bonds.
Question 28.
Write the name of each of the following functional groups: (Foreign 2015, Delhi 2013)
(a) -OH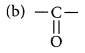
Answer:
(a) -OH : Alcohol![]()
Question 29.
Write the name and molecular formula of the first member of the homologous series of alkynes. (Foreign 2015)
Answer:
General formula for alkyne is CnH2n-2
First member of homologous series of alkyne has the formula, C2H2×2-2 = C2H2 i.e., ethyne.
Question 30.
Define the term functional group. Identify the functional group present in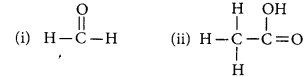
Answer:
An atom or a group of atoms present in a molecule which largely determines its chemical properties, is called functional group.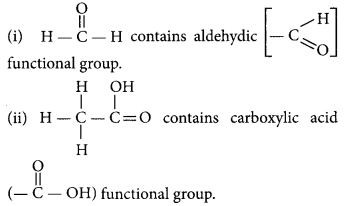
Question 31.
Name the functional group present in each of the following organic compounds:
(i) C2H5Cl
(ii) C2H5OH (Delhi 2012)
Answer:
(i) C2H5Cl contains -Cl (chloro) group which belongs to halo functional group.
(ii) C5H5OH contains -OH group which belongs to alcoholic functional group.
Question 32.
Write the name and formula of the second member of the carbon compounds having functional group -OH. (AI 2012)
Answer:
Those having -OH as functional group belong to alcohol family. Second member of this family is ethanol, C2H5OH.
Question 33.
Write the name and formula of the first member of the series of carbon compounds having functional group (Foreign 2012)
Answer:
Carbon compound containing![]()
group is called carboxylic acid. The first member of this family is methanoic acid (HCOOH).
Question 34.
Butanone is a four-carbon per molecule compound. Name the functional group present in it. (Foreign 2011)
Answer: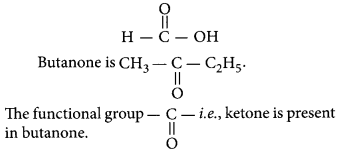
Question 35.
State two properties of carbon which lead to a very large number of carbon compounds. (2/5, AI 2011)
Answer:
Carbon forms a large number of carbon compounds like long chains which may be straight or branched chains or ring of different sizes due to its tetravalency ahd unique property of catenation. Carbon due to its small size forms exceptionally stable compounds by forming strong bonds.
Question 36.
Carbon, a member of group 14, forms a large number of carbon compounds estimated to be about three million. Why is this property not exhibited by other elements of this group? Explain. (2020)
Answer:
Refer to answer 20.
As we move down the group, the element-element bond energies decrease rapidly. For this reason other elements of this group show little or no catenation property.
Question 37.
(a) Why are most carbon compounds poor conductors of electricity?
(b) Write the name and structure of a saturated compound in which the carbon atoms are arranged in a ring. Give the number of single bonds present in this compound. (2018)
Answer:
(a) Due lo catenation, carbon forms covalent bonds with the constituent elements in the carbon compounds, hence it does not have mobile electrons and carbon compounds do not dissociate themselves into ions and hence, they are poor conductor of electricity.
Name: Cyclopentane
Number of single bonds : 15
Question 38.
An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relation between the two in the language of science. (AI 2016)
Answer:
The aldehyde and ketone represented by the
molecular formula, C3H6O.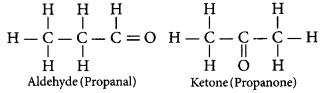
In the language of science, they are called as isomers because both have same molecular formula but different structural formulae (having different functional groups.)
Question 39.
What is meant by isomers? Draw the structures of two isomers of butane, C4H10. Explain why we cannot have isomers of first three members of alkane series. (Delhi 2015, Foreign 2014)
Answer:
Isomers are those molecules which have the same molecular formula but different structural formula i.e., show different properties.
The structures of possible isomers of butane (C4H10) are: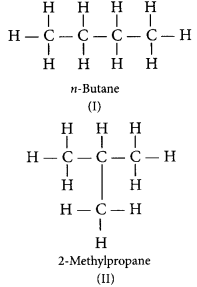
The first three members of alkane series are :
(i) CH4 (methane)
(ii) C2H6 (ethane)
(iii) C3H8 (propane)
In the above members of alkane series, it is not possible to have different arrangements of carbon atoms. Thus, we cannot have isomers of first three members of alkane series.
Question 40.
Write the molecular formula of the following compounds and draw their electron-dot structures:
(i) Ethane
(ii) Ethene
(iii) Ethyne (Foreign 2015)
Answer:
(i) Molecular formula of ethane is C2H6.
Its electron dot structure is :
(ii) Molecular formula of ethene is C2H4. Its electron dot structure is :
(iii) Molecular formula of ethyne is C2H2. Its electron dot structure is :![]()
Question 41.
What is meant by functional group in carbon compounds? Write in tabular form the structural formula and the functional group present in the following compounds :
(i) Ethanol
(ii) Ethanoic acid (Foreign 2015)
Answer:
Refer to answer 30.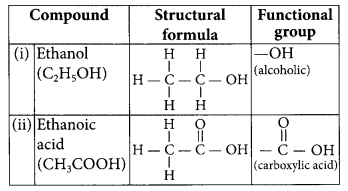
Question 42.
Why is homologous series of carbon compounds so called? Write the chemical formula of two consecutive members of any homologous series and state the part of these compounds that determines their (i) physical and (ii) chemical properties. (Foreign 2015, AI2014, Delhi 2013)
Answer:
Refer to answer 22.
Consecutive members of the homologous series of alcohols are: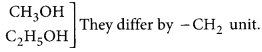
The physical properties are determined by alkyl group/hydrocarbon part/part other than the functional group.
The chemical properties are determined by functional group such as -OH group.
Question 43.
State the meaning of functional group in a carbon compound. Write the functional group present in (i) ethanol and (ii) ethanoic acid and also draw their structures. (Delhi 2014)
Answer:
Refer to answer 30 and 41.
Question 44.
State the meaning of the functional group in an organic compound. Write the formula of the functional group present in alcohols, aldehydes, ketones and carboxylic acids. (Delhi 2014)
Answer:
Refer to answer 30.
The formulae for different functional groups are : alcohols : -OH group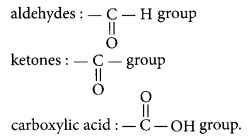
Question 45.
What is meant by homologous series of carbon compounds? Write the general formula of (i) alkenes, and (ii) alkynes. Draw the structures of the first member of each series to show the bonding between the two carbon atoms. (AI 2014)
Answer:
Refer to answer 22.
The general formula for alkenes is CnH2n and for alkynes is CnH2n-2
First member of alkene is ethene, C2H4 and its structure is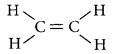
First member of alkyne is ethyne, C2H2 and its structure is H – C ≡ C – H
Question 46.
Define the term structural isomerism’. Explain why propane cannot exhibit this property. Draw the structures of possible isomers of butane, C4H10. (AI 2014)
Answer:
Two or more organic compounds having the same molecular formula but different structures, are called structural isomers and the phenomenon is known as structural isomerism.
There is no possible isomers for propane as it contains three carbon atoms and it is not possible to have different arrangements of these carbon atoms.
Refer to answer 39.
Question 47.
(a) What is a homologous series of compounds? List any two of its characteristics. (Foreign 2011)
(b) What is the next higher homologue of C3H7OH? What is its formula and what is it called? (Foreign 2011)
Answer:
(a) Refer to answer 22.
Two characteristics of homologous series are :
(i) The successive compounds of the homologous series differ by -CH2 unit i.e. 14 mass units.
(ii) Each homologous series belongs to similar class of compounds which shows the same chemical properties.
(b) Next higher homologue of C3H7OH is C4H9OH i.e., butanol.
Question 48.
(a) State the reason why carbon can neither form C4+ cations nor C4- anions, but forms covalent bonds. Also state reasons to explain why covalent compounds
(i) are bad conductors of electricity
(ii) have (ow melting and boiling points.
(b) Write the structural formula of benzene, C6H6. (AI2019)
Answer:
(a) Refer to answer 6.
(i) Refer to answer 3.
(ii) Refer to answer 1.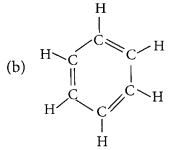
Question 49.
Explain why carbon forms compounds mainly by covalent bond. Explain in brief two main reasons for carbon forming a large number of compounds. Why does carbon form strong bond with most other elements? (Delhi 2015)
Answer:
Refer to answers 6 and 35.
Due to the small size of carbon atom, its nucleus holds the shared pair of electrons between atoms strongly. Thus, carbon forms strong covalent bonds with elements such as hydrogen, oxygen, nitrogen, sulphur, chlorine and other elements.
Question 50.
What are hydrocarbons? Distinguish alkanes from alkenes and each of them from alkynes, giving one example of each. Draw the structure of each compound cited as example to justify your answer. (Foreign 2014)
Answer:
Hydrocarbons are the compounds of carbon and hydrogen atoms. Those hydrocarbons which contain only single carbon-carbon bonds are called alkanes (saturated hydrocarbons) while those having double and triple bonds are called alkenes and alkynes respectively (unsaturated hydrocarbon).
| Alkanes | Alkenes | Alkynes |
| 1. General formula = CnH2n+2 | General formula = CnH2n | General formula = CnH2n-2 |
| 2. Contain C – C single bonds | Contain C = C double bonds | Contain C ≡ C triple bonds |
| 3. e.g., methane (CH4) | e.g., ethene (C2H4) | e.g., ethyne (C2H2) |
Structures of the above examples are: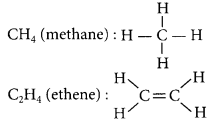
C2H2 (ethyne): H – C ≡ C – H
Question 51.
(a) Define the term‘isomers’.
(b) Draw two possible isomers of the compound with molecular formula C3H6O and write their names.
(c) Give the electron dot structures of the above two compounds. (Delhi 2013)
Answer:
(a) Refer to answer 39.
(b) Two possible isomers of the compound, C3H6O are: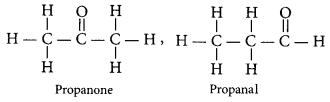
(c) The electron dot structures of propanone and Propanal are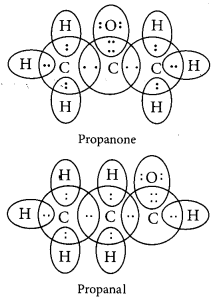
Question 52.
Explain isomerism. State any four characteristics of isomers. Draw the structures of possible isomers of butane, C4H10. (AI 2011)
Answer:
Isomers are those compounds which have same molecular formula but different structures. The phenomenon of existing these isomers are called isomerism.
Four characteristics of isomers are :
(i) They have same molecular formula but different structures.
(ii) For hydrocarbons, isomers is possible only with hydrocarbons having four or more carbon atoms.
(iii) Due to isomerism, a given molecular formula can represent two or more different compounds.
(iv) Due to isomerism, the different compounds have different properties.
Refer to answer 39.
Question 53.
Name the process by which unsaturated fats are changed to saturated fats. (Foreign 2015)
Answer:
Hydrogenation is the process in which unsaturated fats are changed to saturated fats.
Question 54.
Write the chemical equation to show what happen when methane is treated with chlorine in the presence of sunlight ? (1/3, Foreign 2014)
Answer:
When methane is treated with chlorine in the presence of sunlight then substitution reaction takes place. In this, chlorine replaces the hydrogen atom of methane.![]()
Question 55.
Write the respective chemical reaction to show what happens when methane is burnt in presence of oxygen? (1/3, Foreign 2014)
Answer:
When methane is burnt in presence of oxygen then carbon dioxide will be produced.
CH4 + O2 → CO2 + H2O + heat + light
Question 56.
Write one chemical equation to represent the following type of reaction of organic substances: substitution. (1/3, Foreign 2014)
Answer:
Substitution : In this type of reaction one or more hydrogen atoms of a hydrocarbon is replaced by some other atoms.![]()
Question 57.
Give reason for the following : Acetylene burns with a sooty flame. (1/5, Foreign 2011)
Answer:
The formula of acetylene is HC ≡ CH. It is an unsaturated hydrocarbon where carbon content is more than the hydrogen content. Hence, carbon is not completely burnt and the unburnt carbon deposits as a soot.
Question 58.
Give reason for the following : Kerosene does not decolourise bromine water while cooking oils do. (1/5, Foreign 2011)
Answer:
Cooking oils (unsaturated compounds) decolourise bromine water due to formation of addition products whereas kerosene (saturated compound) does not decolourise bromine water.
Question 59.
What happens when 5% alkaline KMnO4 solution is added drop by drop to warm ethanol taken in a test tube? State the role of alkaline KMnO4 solution in this reaction. (2/3, Foreign 2016)
Answer:
When 5% alkaline KMnO4 solution is added drop by drop to warm ethanol then it gets oxidised to ethanoic acid.![]()
Here, alkaline KMnO4 acts as an oxidising agent i.e., the substance which is capable of adding oxygen to others. Thus, alkaline KMnO4 provides oxygen to ethanol to form ethanoic acid.
Question 60.
3 mL of ethanol is taken in a test tube and warmed gently in a water bath. A 5% solution of alkaline potassium permanganate is added first drop by drop to this solution, then in excess.
(i) How is 5% solution of KMnO4 prepared?
(ii) State the role of alkaline potassium permanganate in this reaction. What happens on adding it in excess?
(iii) Write chemical equation of this reaction. (2020)
Answer:
(i) 5% solution of KMnO4 is prepared by adding 5 g of KMnO4 in 95 g of water.
(ii) Here alkaline KMnO4 acts as an oxidising agent. It oxidises ethanol to ethanoic acid by donating nascent oxygen. If excess of KMnO4 is added the purple colour will persist indicating no more alcohol is left and there is no reaction.![]()
Question 61.
Two carbon compounds X and Y have the molecular formula C4H8 and C5H12 respectively. Which one of these is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case. (Delhi 2017)
Answer:
All unsaturated hydrocarbons (containing double or triple bonds) have tendency to get converted to saturated hydrocarbons (single bonds) by adding small molecules such as hydrogen (H2), halogens (X2), etc. Such reactions are called addition reactions.
Compound X i.e., C4H8 belongs to alkene series (CnH2n) while compound Y i.e., C5H12 belongs to alkane series (CnH2n+2). Thus, compound X will undergo addition reaction.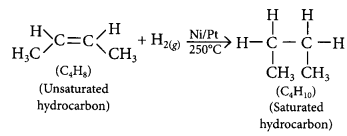
Question 62.
The molecular formula of two carbon compounds are C4H8 and C3H8. Which one of the two is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case. (Delhi 2017)
Answer:
C3H8 belongs to alkane series (CnH2n+2) Refer to answer 61.
Question 63.
What is an oxidising agent? What happens when an oxidising agent is added to propanol? Explain with the help of a chemical equation. (Delhi 2016)
Answer:
The substance that supply oxygen in a reaction for oxidation is called oxidising agent e.g., potassium permanganate, potassium dichromate, etc.
When propanol is heated with alkaline KMnO4, it gets oxidised to propanoic acid.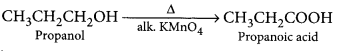
Question 64.
Draw the electron-dot structure for ethyne. A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose? (AI 2015)
Answer:
The formula for ethyne is C2H2 and its electron dot structure is :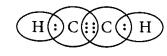
A mixture of ethyne and oxygen is burnt for welding so that complete oxidation of ethyne takes place. If in place of oxygen, air is taken which contains less amount of oxygen then incomplete combustion of oxygen takes place and temperature required for welding will not be attained.
Question 65.
Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential condition for an addition reaction. Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction. (AI 2015, Delhi 2014)
Answer:
Alkenes, having general formula as CnH2n and alkynes, having general formula as CnH2n-2 are the class of hydrocarbons in which addition reaction is possible.
The essential conditions for addition reaction are :
(i) Presence of unsaturated hydrocarbon.
(ii) Presence of catalyst such as Ni/Pt/Pd.
Let us take an example of ethene. It undergoes addition reaction with hydrogen when it is heated in the presence of nickel catalyst to form ethane. The reaction is known as hydrogenation.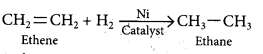
Question 66.
Why are certain compounds called hydrocarbons? Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction that converts alkenes into alkanes and also write a chemical equation to show the necessary conditions for the reaction to occur. (AI 2017)
Answer:
Refer to answers 50 and 65.
Question 67.
What are hydrocarbons? Write the name and general formula of
(i) saturated hydrocarbons
(ii) unsaturated hydrocarbons, and draw the structure of one hydrocarbon of each type. How can an unsaturated hydrocarbon be made saturated? (AI 2012)
Answer:
Refer to answer 50.
Unsaturated hydrocarbons can be made to saturated hydrocarbons by hydrogenation reaction using nickel (Ni) as a catalyst.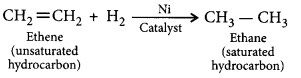
Question 68.
(a) With the help of a suitable example, explain the process of hydrogenation mentioning the conditions of the reaction and any one change in physical property with the formation of the product. (Delhi 2015, 2013, Foreign 2012)
(b) How does a saturated hydrocarbon react with chlorine? Write chemical equation for it. What type of reaction is it called and why? (Foreign 2012)
Answer:![]()
groups that can be hydrogenated.
Hydrogenation is the addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydrocarbon.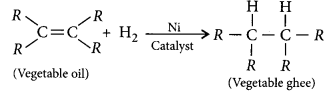
Here R can be any alkyl group.
There is the change of unsaturated compound from the liquid state to saturated compound in the solid state thus, melting point increases.
(b) Saturated hydrocarbon reacts with chlorine to form a substituted product, e.g.,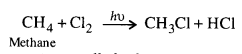
This reaction is called substitution reaction as here one hydrogen of methane is substituted by one chlorine atom.
Question 69.
Assertion (A) : Esterification is a process in which a sweet smelling substance is produced.
Reason (R): When esters react with sodium hydroxide, an alcohol and sodium salt of carboxylic acid are obtained.
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true. (2020)
Answer:
(b): When an ester reacts with the base saponification reaction occurs.
Question 70.
Assertion (A) : Ethanoic acid is also known as glacial acetic acid.
Reason (R) : The melting point of pure ethanoic acid is 290 K and hence it often freezes during winters in cold climates.
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true. (2020)
Answer:
(a): Pure ethanoic acid or acetic acid freezes below room temperature into white crystals that resemble glaciers.
Question 71.
Draw the structure for ethanoic acid molecule, CH3COOH. (AI 2011)
Answer:
Structure of ethanoic acid is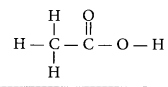
Question 72.
A compound ‘X’ on heating with excess cone, sulphuric acid at 443 K gives an unsaturated compound ‘Y’. ‘X’ also reacts with sodium metal to evolve a colourless gas ‘Z’. Identify ‘X’, ‘Y’ and ‘Z’. Write the equation of the chemical reaction of formation of ‘Y’ and also write the role of sulphuric acid in the reaction. (2018)
Answer:
As X reads with cone. H2SO4 to give an alkene so it should be an alcohol as cone. H2SO4 acts as a dehydrating agent. The reaction of X with Na also confirms that it is an alcohol because alcohols react with Na metal to evolve colourless hydrogen gas.![]()
Here, conc. H2SO4 acts as a dehydrating agent i.e., helps in the removal of water.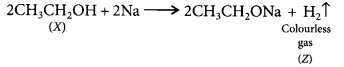
Question 73.
Write the chemical equations to show what happens when
(i) an ester reacts with a base?
(ii) ethanol reacts with ethanoic acid in the presence of sulphuric acid? (2/3, Foreign 2014)
Answer:
(i) When an ester reacts with the base then it gives sodium salt of carboxylic acid and an alcohol. It is known as saponification reaction.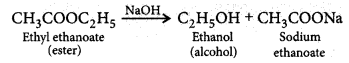
(ii) Carboxylic acids react with alcohols in the presence of a little concentrated sulphuric acid to form pleasant smelling esters. This reaction is called esterification reaction.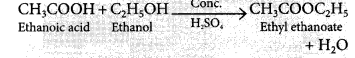
Question 74.
Write the respective chemical equations to show what happens when
(i) ethanol is heated with concentrated sulphuric acid at 443 K ?
(ii) ethanol reacts with ethanoic acid in the presence of an acid acting as a catalyst? (2/3, Foreign 2014)
Answer: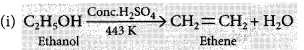
(ii) Refer to answer 73(ii).
Question 75.
Write one chemical equation to represent each of the following types of reactions of organic substances:
(i) Esterification
(ii) Saponification (2/3, Delhi 2011)
Answer:
(i) Refer to answer 73(ii).
(ii) Refer to answer 73(i).
Question 76.
Complete the following chemical equations : (Delhi 2017)
(i) CH3COOC2H5 + NaOH →
(ii) CH3COOH + NaOH →![]()
Answer: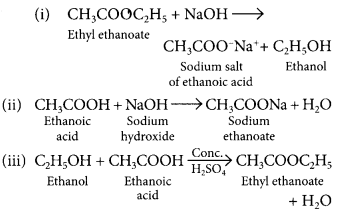
Question 77.
Complete the following chemical equations: (Delhi 2017)
(i) C2H5OH + O2 →![]()
(iii) CH3COOH + NaHCO3 →
Answer:
(ii) Refer to answer 74(i).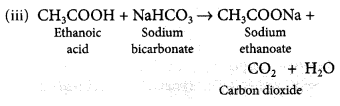
Question 78.
Write the structural formula of ethanol. What happens when it is heated with excess of cone. H2SO4 at 443 K? Write the chemical equation for the reaction stating the role of cone. H2SO4 in this reaction. (AI 2017, Delhi 2015, 2013)
Answer:
The structural formula of ethanol (C2H5OH) is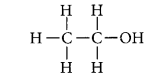
When ethanol is heated with conc. H2SO4 at 443 K then it looses a water molecule to form unsaturated alkene (ethene) as a product.![]()
Here conc. H2SO4 acts as a dehydrating agent i.e., helps in the removal of water.
Question 79.
What happens when (write chemical equation in each case)
(a) ethanol is burnt in air?
(b) ethanol is heated with excess cone. H2SO4 at 443 K?
(c) a piece of sodium is dropped into ethanol? (AI 2017)
Answer:
(a) Refer to answer 77(i).
(b) Refer to answer 74(i).
(c) When a small piece of sodium is dropped into ethanol then hydrogen gas is liberated which burns with a pop sound.
2C2H5OH + 2Na → 2C2H5O–Na+ + H2 ↑
Question 80.
Distinguish between esterification and saponification reaction with the help of the chemical equations for each. State one use of each (i) esters, and (ii) saponification process. (AI 2017, Foreign 2012)
Answer: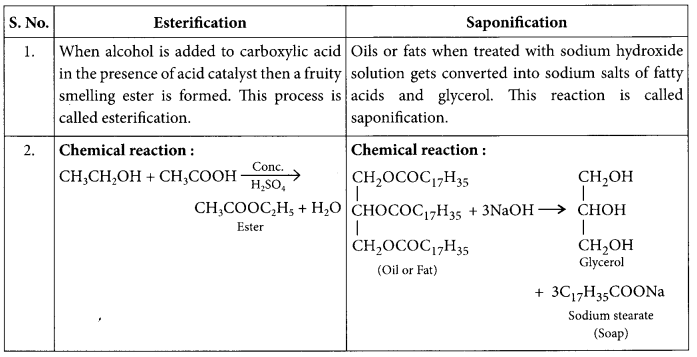
Use of esters: They are used for making perfumes or used as artificial flavouring substances.
Use of saponification process : This process is used in making soaps.
Question 81.
Explain esterification reaction with the help of a chemical equation. Describe an activity to show esterification. (AI 2017)
Answer:
Refer to answer 80.
Aim : To demonstrate esterification process using ethanol and acetic acid.
Materials required : Beaker, water, test tube, ethanol, acetic acid, cone. H2SO4, tripod stand, burner, wire gauze, etc.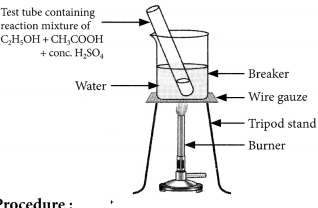
Procedure:
– Take 2 mL of ethanol in a test tube.
– Take 2 mL of ethanoic acid (acetic acid) into it.
– Add few drops of cone. H2SO4
– Warm it in a beaker containing water.
– Observe the smell of the products formed. Observations: Pleasant fruity smelling compound (called ester) is formed.
Chemical reaction: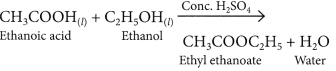
Conclusion : Carboxylic acid reacts with alcohol in presence of cone. H2SO4 which acts as a dehydrating agent to form esters.
Question 82.
When ethanol reacts with ethanoic acid in the presence of cone. H2SO4, a substance with fruity smell is produced. Answer the following:
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of cone. H2SO4 in this reaction. (Delhi 2016)
Answer:
(i) When ethanol reacts with ethanoic acid in presence of cone. H2SO4, ethyl ethanoate is formed which belongs to the class of ester compounds, having fruity smell.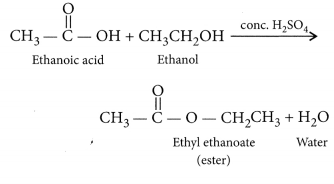
(ii) The above reaction is called esterification which occurs in presence of cone. H2SO4 which acts as a dehydrating agent and helps in the removal of water. Cone. H2SO4 also acts as a catalyst to speed up the reaction.
Question 83.
Name the compound formed when ethanol is heated in excess of cone, sulphuric acid at 443 K. Also write the chemical equation of the reaction stating the role of cone, sulphuric acid in it. What would happen if hydrogen is added to the product of this reaction in the presence of catalyst such as palladium or nickel? (Delhi 2016, Foreign 2015)
Answer:
Refer to answer 78.
If hydrogen is added to ethene in presence of palladium or nickel catalyst then one atom of hydrogen adds to each carbon atom of ethene to form ethane.![]()
Question 84.
Write chemical equation of the reaction of ethanoic acid with the following :
(a) Sodium;
(b) Sodium hydroxide;
(c) Ethanol
Write the name of one main product of each reaction. (AI 2016)
Answer:
Ethanoic acid reacts with sodium as well as sodium hydroxide to form sodium ethanoate.
(b) Refer to answer 76(ii).
(c) Refer to answer 76(iii).
Question 85.
On dropping a small piece of sodium in a test tube containing carbon compound ‘X’ with molecular formula C2H6O, a brisk effervescence is observed and a gas ‘Y’ is produced. On bringing a burning splinter at the mouth of the test tube the gas evolved burns with a pop sound. Identify ‘X’ and ‘Y’. Also write the chemical equation for the reaction. Write the name and structure of the product formed, when you heat ‘X’ with excess cone, sulphuric acid. (AI 2016)
Answer:
Ethanol reacts with sodium to form sodium ethoxide and hydrogen gas is liberated which burns with a pop sound.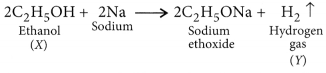
Thus, compound X is ethanol and gas Y is hydrogen gas.
When ethanol is heated with excess of concentrated sulphuric acid then it gets dehydrated to form ethene.![]()
Question 86.
Write three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate. Write balanced chemical equation in each case. Write the name of the reactants and the products other than ethanoic acid and sodium ethanoate in each case. (AI 2016)
Answer:
Ethanoic acid reacts with Na2CO3 to form sodium ethanoate and CO2 gas is liberated.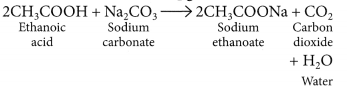
With sodium hydrogen carbonate it forms sodium ethanoate.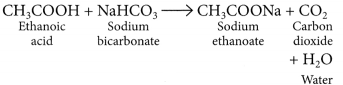
With NaOH it forms sodium ethanoate.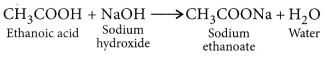
Question 87.
Write the nam e and molecular formula of an organic compound having its name suffixed with ‘ol’ and having two carbon atoms in its molecule. Write balanced chemical equation to indicate what happens when this compound is heated with excess cone. H2SO4 and the narpe of main product formed. Also state the role of cone. H2SO4 in the reaction. (Foreign 2016)
Answer:
Those organic compounds having suffix ‘oF are alcohols. As the alcohol is having two carbon atoms in its molecule so, it is ethanol.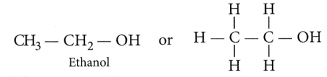
Refer to answer 78.
Question 88.
An organic compound ‘P’ is a constituent of wine. ‘P’ on reacting with acidified K2Cr2O7 forms another compound ‘Q’. When a piece of sodium is added to ‘Q’, a gas ‘R’ evolves which burns with a pop sound. Identify P, Q and R and write the chemical equations of the reactions involved. (Foreign 2016)
Answer:
‘P’ is ethanol which is a constituent of wine. Ethanol on reacting with acidified potassium dichromate (K2Cr2O7) solution gives ethanoic acid ‘Q’.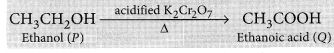
When a piece of sodium is added to ethanoic acid then sodium salt of ethanoic acid is formed with the liberation of hydrogen gas which burns with a pop sound.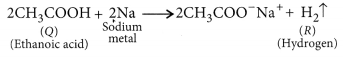
Question 89.
List two tests for experimentally distinguishing between an alcohol and a carboxylic acid and describe how these tests are performed. (AI 2015)
Answer:
Tests for distinguishing between an alcohol and a carboxylic acid are :
(i) Litmus test : When we place a drop of carboxylic acid on blue litmus paper it turns red while alcohol will not change the colour of blue litmus paper.
(ii) Sodium hydrogen carbonate test/sodium carbonate test: If a pinch of NaHCO3 or Na2CO3 is added to two test tubes containing alcohol and carboxylic acid respectively, then test tube containing carboxylic acid will show the evolution of colourless gas with brisk effervescence while test tube containing alcohol does not show any reaction.
Question 90.
What are esters? How are they prepared? List two uses of esters. (Delhi 2014)
Answer:
Esters are generally volatile liquids which have pleasant fruity smell.
Esters are prepared when a carboxylic acid reacts with an alcohol in the presence of small amount of concentrated H2SO4. For example, when ethanoic acid reacts with ethanol it forms an ester (i.e. ethyl ethanoate).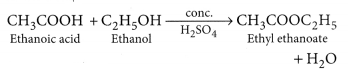
Uses of ester:
1. It is used in making perfumes.
2. It is used in making artificial flavours and essences used in ice-creams, sweets and cold drinks.
Question 91.
A carboxylic acid (molecular formula, C2H4O2) reacts with an alcohol in the presence of an acid catalyst to form a compound ‘X’. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid C2H4O2. Write the name and structure of (i) carboxylic acid, (ii) alcohol and (iii) the compound ‘X’ (AI 2014)
Answer:
The molecular formula of carboxylic acid is C2H4O2. Thus, it should be acetic acid (ethanoic acid).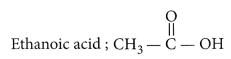
It reacts with alcohol in presence of acid catalyst to give compound ‘X’.
As alcohol on oxidation with alkaline KMnO4 gives the same acid i.e. ethanoic acid, hence alcohol must contain two carbon atoms. Thus, formula for alcohol is CH3CH2OH i.e. ethanol.
Reactions involved are: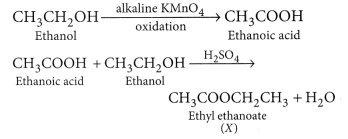
(i) Structure of ethanoic acid :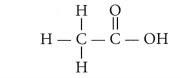
(ii) Structure of ethanol:
(iii) Structure of ethyl ethanoate (X):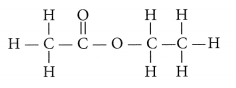
Question 92.
Write the chemical equation to explain what happens when ethanol is heated with alkaline solution, of potassium permanganate. Mention two physical properties and two uses of ethanol. (Foreign 2014)
Answer:
When ethanol is heated with alkaline solution of potassium permanganate then oxidation of ethanol takes place to form ethanoic acid.
Two physical properties of ethanol are:
1. It is liquid at room temperature.
2. It is soluble in water in all proportions.
Two uses of ethanol are :
1. It is used as a liquor for drinking purpose.
2. It is a good solvent and hence, it is used in medicines such as tincture of iodine, cough syrup and many tonics.
Question 93.
Write chemical equations to describe two examples of different oxidations of ethanol. List two uses of ethanol. (Foreign 2014)
Answer:
Addition of oxygen to any substance is called oxidation.
Ethanol gets oxidised to ethanoic acid as :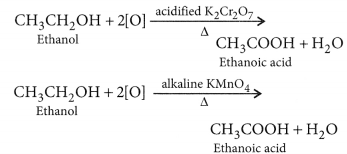
Refer to answer 92.
Question 94.
Write the chemical equations to show what happens when
(i) sodium hydroxide is added to ethanoic acid?
(ii) solid sodium hydrogen carbonate is added to ethanoic acid?
(iii) ethanol reacts with sodium? (Foreign 2014)
Answer:
(i) Refer to answer 76(ii).
(ii) Refer to answer 77(iii).
(iii) Refer to answer 79(c).
Question 95.
Write chemical equations for what happens when
(i) sodium metal is added to ethanoic acid?
(ii) solid sodium carbonate is added to ethanoic acid?
(iii) ethanoic acid reacts with a dilute solution of sodium hydroxide? (AI 2011)
Answer:
(i) Refer to answer 84(a).
(ii) Refer to answer 86.
(iii) Refer to answer 76(ii).
Question 96.
(a) What is a homologous series? Explain with an example.
(b) Define the following terms giving one example of each.
(i) Esterification (ii) Addition reaction (2020)
Answer:
(a) Refer to answer 22.
For example, alkane series has general formula CnH2n + 2.
First member of homologous series of alkane is .methane, i.e., CH4.
Second member of homologous series of alkane is ethane, i.e., C2H6.
Third member of homologous series of alkane is propane i.e., C3H8.
(b) (i) Refer to answer 73(ii).
(ii) Addition reactions : Those reactions in which atoms or group of atoms are simply added to a double or triple bond without the elimination of any atom or molecule, are known as addition reactions.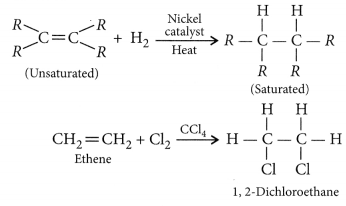
Question 97.
(a) Carry out following conversions :
(i) Ethanol to ethene
(ii) Ethanol to ethanoic acid
(b) Differentiate between addition reaction and substitution reaction. Give one example of each. (2020)
Answer:
(a) (i) When ethanol is heated with cone. H2SO4 at 443 K, ethene is obtained due to dehydration of ethanol.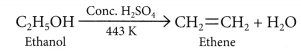
(ii) When 5 % alkaline KMnO4 solution is added drop by drop to warm ethanol then it gets oxidised to ethanoic acid.![]()
(b) Refer to answer 96(ii).
Substitution reactions : The reactions which involve the displacement or substitution of an atom or a group of atoms in an organic compound by another atom or group of atoms, are known as substitution reactions.
Saturated hydrocarbons are fairly unreactive and inert in the presence of most of the reagents. However, in presence of sunlight, hydrocarbons undergo rapid substitution reactions, e.g.,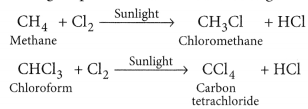
Question 98.
Write the chemical formula and name of the compound which is the active ingredient of all alcoholic drinks. List its two uses. Write chemical equation and name of the product formed when this compound reacts with
(i) sodium metal
(ii) hot concentrated sulphuric acid. (Delhi 2019)
Answer:
Ethanol having chemical formula C2H5OH is the active ingredient of all alcoholic drinks.
Uses of ethanol:
1. Ethanol is widely used in industry as a solvent.
2. Ethanol is used as an antiseptic for wounds in the form of rectified spirit.
Chemical equations:
(i) Refer to answer 79(c).
(ii) Refer to answer 74(i).
Question 99.
(a) Define the term isomer.
(b) Two compounds have same molecular formula C3H6O. Write the name of these compounds and their structural formula.
(c) How would you bring the following conversions:
(i) Ethanol to ethene
(ii) Propanol to propanoic acid? (AI 2019)
Answer:
(a) Refer to answer 39.
(b) Refer to answer 51 (b).
(c) (i) Refer to answer 97(a)(i).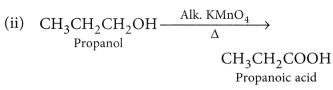
Question 100.
A carbon compound ‘P’ on heating with excess cone. H2SO4 forms another carbon compound ‘Q’ which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound ‘R’ One molecule of ‘R’ on combustion forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved. (AI 2016)
Answer:
When ethanol is heated with excess of concentrated H2SO4 it gets dehydrated to form ethene.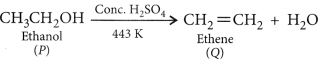
When ethene is heated with hydrogen in presence of nickel catalyst it forms ethane.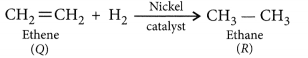
Ethane on oxidation gives two moles of carbon dioxide and three moles of water.
CH3CH3 +
Question 101.
List in tabular form three physical and two chemical properties on the basis of which ethanol and ethanoic acid can be differentiated. (Delhi 2012)
Answer:
| Ethanoic acid (Physical properties) | Ethanol (Physical properties) |
| (i) It has moderate melting point (290 K) and boiling point (391 K). | It has very low melting point (156 K) and low boiling point (351 K). |
| (ii) It has a sour taste. | It has a burning taste. |
| (iii) It has a pungent smell. | It has a distinct smell. |
(ii)
| Chemical properties | Chemical properties |
| (i) It is acidic in nature and turns blue litmus to red. | It is neutral in nature and thus, it does not turn blue litmus to red or vice-versa. |
| (ii) Ethanoic acid reacts with Na2CO3 or NaHCO3 to give brisk effervescence of CO2 gas. 2CH3COOH + Na2CO3 → 2CH3COONa + CO2 ↑+ H2O | Ethanol does not react with Na2CO3 or NaHCO3, C2H5OH + Na2CO3 → No reaction |
Question 102.
(a) In a tabular form, differentiate between ethanol and ethanoic acid under the following heads:
(i) Physical state
(ii) Taste
(iii) NaHCO3 test
(iv) Ester test
(b) Write a chemical reaction to show the dehydration of ethanol. (Delhi 2011)
Answer:
(a)
(b) Refer to answer 97(a)(i).
Question 103.
Several factories were pouring their wastes in rivers A and B. Water samples were collected from these two rivers. It was observed that sample collected from river A was acidic while that of river B was basic. The factories located near A and B are
(a) Soaps and detergents factories near A and alcohol distillery near B.
(b) Soaps and detergents factories near B and alcohol distillery near A.
(c) Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
(d) Lead storage battery manufacturing factories near B and soaps and detergents factories near A. (2020)
Answer:
(c) Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
Question 104.
Why does micelle formation take place when soap is added to water? Why are micelles not formed when soap is added to ethanol? (3/5, AI 2011)
Answer:
A soap molecule has two ends with different properties, one end is polar i.e., water soluble or hydrophilic while other end is non-polar i.e., water insoluble or hydrophobic. When soap is added to water, the polar ends get dissolve in water and non-polar ends get dissolved in each other and directed towards the centre. As a result, a spherical ionic molecule known as micelles, formation takes place. Since, soaps are soluble in ethanol, therefore, micelles formation does not occur.
Question 105.
Soaps and detergents are both, types of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps. (Delhi 2017, AI 2015)
Answer:
Soaps are the sodium or potassium salts of higher fatty acids. The ionic group in soaps is -COO–Na+.
On the other hand, synthetic detergents are the sodium salts of a long chain alkylbenzenesulphonic acids or long chain alkyl hydrogen sulphates. The ionic group in synthetic detergents is
-SO3– Na+ or -OSO3 –Na+
Cleansing action of soap :
A soap molecule contains a polar part (COO–Na+) called polar end and a non-polar part consisting of a long chain carbon atoms. This part is called hydrocarbon end.
The polar end is water soluble whereas hydrocarbon part is water-repellent and oil soluble.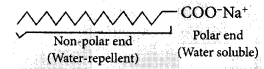
When an oily (dirty) piece of cloth is put into soap solution, the hydrocarbon part of the molecule attaches itself to the oily drop and the -COO– end orients itself towards water. Na+ ions in solution arrange themselves around the -COO– ions. The negatively charged micelle so formed entraps the oily dirt. The negatively charged micelle repel each other due to the electrostatic repulsion. As a result, the tiny oily dirt particles do not come together and get washed away in water during rinsing.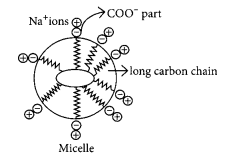
In hard water, soap does not form lather as hard water contains Ca2+and Mg2+ ions. Soap reacts with these ions to form insoluble calcium and magnesium salts of fatty acids.![]()
Two problems which arise due to the use of detergents instead of soaps are :
(i) Synthetic detergents are non-biodegradable and hence, cause water pollution.
(ii) Synthetic detergents also cause skin related problems.
Question 106.
What are micelles? Why does it form when soap is added to water? Will a micelle be formed in other solvents such as ethanol also? State briefly how the formation of micelles help to clean the clothes having oily spots. (Foreign 2016)
Answer:
Refer to answer 104.
Refer to answer 105.
Question 107.
(a) You have three unlabelled test tubes containing ethanol, ethanoic acid and soap solution. Explain the method you would use to identify the compounds in different test tubes by chemical tests using litmus paper and sodium metal.
(b) Give the reason of formation of scum when soaps are used with hard water. (Foreign 2016)
Answer:
(a) The tests may be tabulated as below:
| s.no. | Solution | Blue litmus paper | Red litmus paper | Sodium Metal |
| 1. | Ethanol | No change | No change | Hydrogen gas |
| 2. | Ethanoic acid | Turns red | No change | Hydrogen gas |
| 3. | Soap solution | No change | Turns blue | No reaction |
(b) Hard water contains hydrogen carbonates, chlorides and sulphates of calcium and magnesium. When soap is added to hard water it reacts with these salts to form scum which is insoluble in water and floats on the top of the water surface. The scum is formed due to the formation of insoluble calcium or magnesium salts of fatty acids.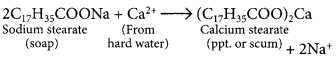
Question 108.
What is the difference between the molecules of soaps and detergents, chemically? Explain the cleansing action of soaps. (Delhi 2015)
Answer:
Refer to answer 105.
Question 109.
What is the difference between the chemical composition of soaps and detergents? State in brief the action of soaps in removing an oily spot from a shirt. Why are soaps not considered suitable for washing where water is hard? (Delhi 2012)
Answer:
Refer to answers 105 and 107(b).
Question 110.
What are detergents chemically? List two merits and two demerits of using detergents for cleansing. State the reason for the suitability of detergents for washing, even in the case of water having calcium and magnesium ions. (AI 2012)
Answer:
Detergents are generally ammonium or sulphonate or sulphate salts of long chain carboxylic acids. The more common detergents are sodium salts of long chain alkyl benzene sulphonic acids.
Merits of using detergents :
(i) Detergents are very strong cleansing agents.
(ii) They can form lather well even in hard water as they do not form insoluble calcium or magnesium salts.
Demerits of using detergents :
(i) As detergents are sodium salts of long chain alkyl benzene sulphonic acids which are very bulky molecules, are not easily degraded by bacteria and hence, they are non-biodegradable.
(ii) They are highly basic in nature and cause damage to skin.
Synthetic detergents can be used even in hard water because they do not react with Ca2+ and Mg2+ ions present in hard water. They do not form curdy white precipitates (scum) of calcium and magnesium salts of fatty acids.
Question 111.
What are soaps and detergents chemically? Explain the action of cleaning by soaps. State the reason why we can wash our clothes even in hard water using detergents. (Foreign 2012)
Answer:
Refer to answers 105 and 110.
Question 112.
(a) What is a soap? Why are soaps not suitable for washing clothes when the water is hard?
(b) Explain the action of soap in removing an oily spot from a piece of cloth. (Delhi 2011)
Answer:
(a) Refer to answers 105 and 107(b).
(b) Refer to answer 105.
Question 113.
(a) What is a detergent? Name one detergent.
(b) Write two advantages and two dis-advantages of using detergents over soaps.
(c) Why, by using a detergent, can we wash clothes even in hard water?
Answer:
(a) Detergents are ammonium or sulphonate or sulphate salts of long chain hydrocarbons containing 12-18 carbon atoms e.g., dodecyl benzene sulphonate.
(b) Refer to answer 110.
(c) Refer to answer 110.
Carbon and its Compounds Notes CBSE Class 10 Science Chapter 4
Bonding in Carbon: The Covalent bond, Electron dot structure, Physical properties of organic compounds, Allotropes of Carbon.
Covalent Bond: The atomic number of carbon is 6. Its electronic configuration is 2, 4. It requires, 4 electrons to achieve the inert gas electronic configuration. But carbon cannot form an ionic bond
It could gain four electrons forming C4- cation. But it would be difficult for the nucleus with six protons to hold on to ten electrons.
It could lose four electrons forming C4+ cations. But it requires a large amount of energy to remove four electrons.
Thus, carbon overcomes this problem by sharing of its valence electrons with other carbon atoms or with atoms of other elements.
The bond formed by mutual sharing of electron pairs between two atoms in a molecule is known as Covalent Bond.
Types of Covalent Bond:
- Single Covalent Bond: When a single pair of electrons are shared between two atoms in a molecule. For example; F2, Cl2, H2 etc.
- Double Covalent Bond: When two pairs of electrons are shared between two atoms in a molecule. For example; O2, CO2 etc.
- Triple Covalent Bond: When three pairs of electrons are shared between two atoms in a molecule. For example; N2 etc.
Electron Dot Structure: The electron dot structures provides a picture of bonding in molecules in terms of the shared pairs of electrons and octet rule.
Formation of Hydrogen Molecule
Atomic number of Hydrogen = 1
Number of valence electrons = 1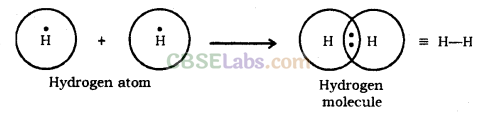
Formation of CH4 Molecule
Atomic number of Carbon = 6 [2, 4]
Number of valence electrons = 4
Atomic number of Hydrogen = 1
Number of valence electrons = 1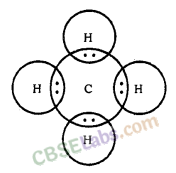
Formation of CO2 Molecule
Atomic number of Carbon = 6 [2, 4]
Number of valence electrons = 4
Atomic number of Oxygen = 8 [2, 6]
Number of valence electrons = 6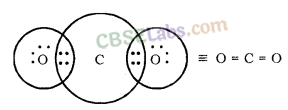
Formation of H2S Molecule
Atomic number of Sulphur = 16 [2, 8, 6]
Number of valence electrons = 6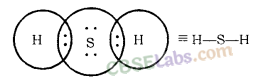
Physical Properties of Organic Compounds
Most of the organic compounds have low boiling and melting point, due to the weak force of attraction (i.e., the inter-molecular force of attraction) between these molecules.
Most carbon compounds are poor conductors of electricity, due to the absence of free electrons and free ions.
| Compounds | M.P. (K) | B.P. (K) |
| Acetic acid (CH3COOH) | 290 | 391 |
| Chloroform (CHCl3) | 209 | 334 |
| Ethanol (CH3CH2OH) | 156 | 351 |
| Methane (CH4) | 90 | 111 |
Allotropes of Carbon
Allotropy: The phenomenon in which the element exists in two or more different physical states with similar chemical properties are called Allotropy.
Carbon has Three Main Allotropes
- Diamond: In this, carbon, an atom is bonded to four other atoms of carbon forming three-dimensional structures. It is the hardest substance and an insulator. It is used for drilling rocks and cutting. It is also used for making jewellery.
- Graphite: In this, each carbon atom is bonded to three other carbon atoms. It is a good conductor of electricity and used as a lubricant.
- Buckminster Fullerene: It is an allotrope of the carbon-containing cluster of 60 carbon atoms joined together to form spherical molecules. It is dark solid at room temperature.
Versatile nature of Carbon, Hydrocarbons, Isomerism, Homologous series, Functional groups, Nomenclature of functional groups.
Versatile Nature of Carbon: The existence of such a large number of organic compounds is due to the following nature of carbon,
- Catenation
- Tetravalent nature.
(i) Catenation: The self linking property of an element mainly carbon atom through covalent bonds to form long straight, branched and rings of different sizes are called Catenation.
This property is due to
- The small size of the carbon atom.
- The great strength of the carbon-carbon bond.
Carbon can also form stable multiple bonds (double or triple) with itself and with the atoms of other elements.
Straight Chain
Branched Chain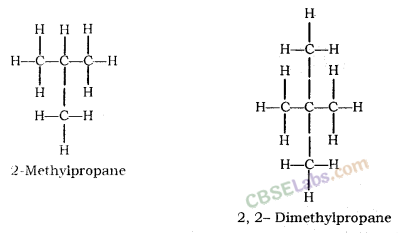
Rings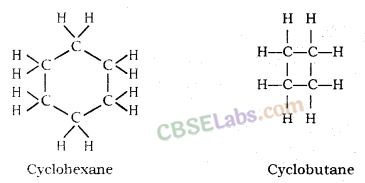
(ii) Tetravalent Nature: Carbon has valency of four. It is capable of bonding with four other atoms of carbon or some other heteroatoms with single covalent bond as well as double or triple bond.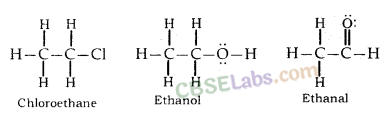
Hydrocarbons: Compounds of carbon and hydrogen are known as hydrocarbons.
For example; Methane (CH4), Ethane (C2H6), Ethene (C2H4), Ethyne (C2H2) etc.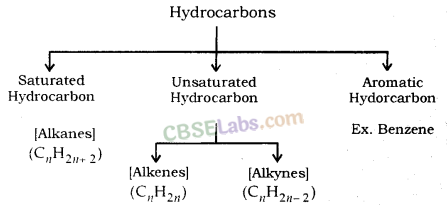
Saturated Hydrocarbon (Alkanes): General formula is CnH2n+2.
n = number of carbon atoms.
In this, the carbon atoms are connected by only a single bond.
For example; Methane (CH4), Ethane (C2H6) etc.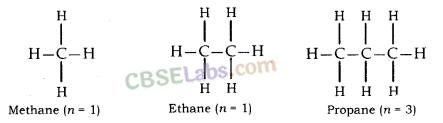
Unsaturated Hydrocarbons
Alkenes: General formula is CnH2n, where n = number of carbon atoms.
In this, the two carbon atoms are connected by double bond.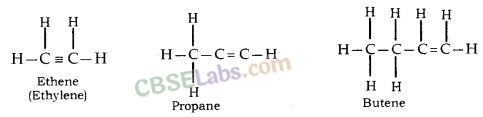
Alkynes: General formula is CnH2n-2, where n = number of carbon atoms. In this, the two carbon atoms are connected by triple bond.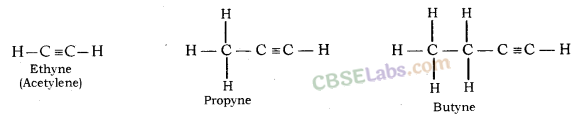
Electron Dot Structure of Hydrocarbons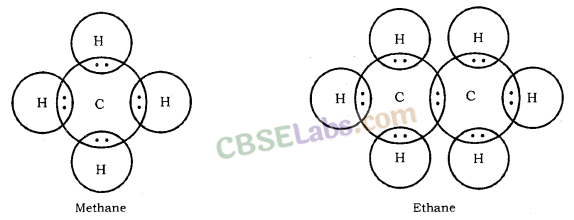
Isomerism: Compounds having the same molecular formula but different structural formula and properties are known as Isomers and this phenomenon is known as Isomerism.
Structural Isomerism: Compounds having the same molecular formula but different structures are called Structural isomers. Example: Isomers of butane (C4H10)
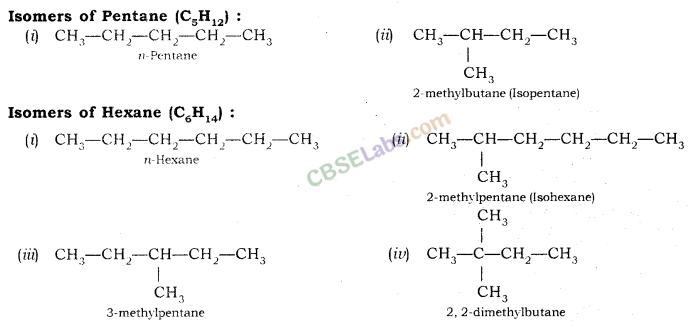
Homologous Series: Series of organic compounds having the same functional group and chemical properties and successive members differ by a CH2 unit or 14 mass units are known as Homologous series.
Homologous series of Alkanes, Alkenes and Alkynes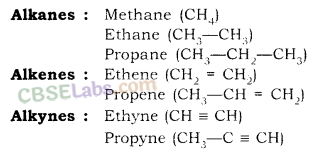
Characteristic of Homologous Series
- The successive members in homologous series differ by CH2 unit or 14 mass unit.
- Members of given homologous series have the same functional group.
- All the members of homologous series shows similar chemical properties.
Functional Group: An atom or group of atoms present in a molecule which largely determines its chemical properties are called Functional Group.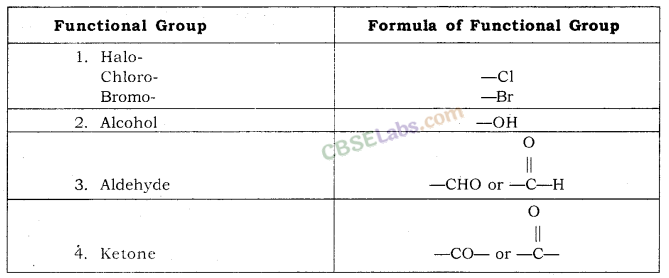
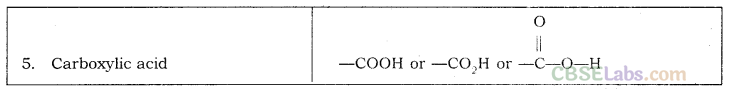
Nomenclature of Organic Compounds: It is difficult to remember millions of compounds by their individual common name. Thus, to systematize the nomenclature of organic compounds IUPAC (International Union of Pure and Applied Chemistry) has given certain rule which is as follows:
1. Identify the Number of Carbon Atoms in the Compound
| S. No | Number of Carbon Atoms | Word Root (-) (Suffix) | Single bond |
| 1. | One carbon atoms (1-C) | Meth | + ane |
| 2. | Two carbon atoms (2-C) | Eth | + ane |
| 3. | Three carbon atoms (3-C) | Prop | + ane |
| 4. | Four carbon atoms (4-C) | But | + ane |
| 5. | Five carbon atoms (5-C) | Pent | + ane |
| 6. | Six carbon atoms (6-C) | Hex | + ane |
2. Identify the functional group
| S. No. | Functional Group | Prefix | Suffix |
| 1. | Double bond (=) | — | ene |
| 2. | Triple bond (≡) | — | yne |
| 3. | Chlorine (—Cl) | Chloro | — |
| 4. | Bromine (—Br) | Bromo | — |
| 5. | Alcohol (-OH) | — | ol |
| 6. | Aldehyde (-CHO) | — | al |
| 7. | Ketone (-CO-) | — | one |
| 8. | Carboxylic acid (-COOH) | — | oic acid |
3. Name the Compounds By Following Order
Prefix + Word Root + Suffix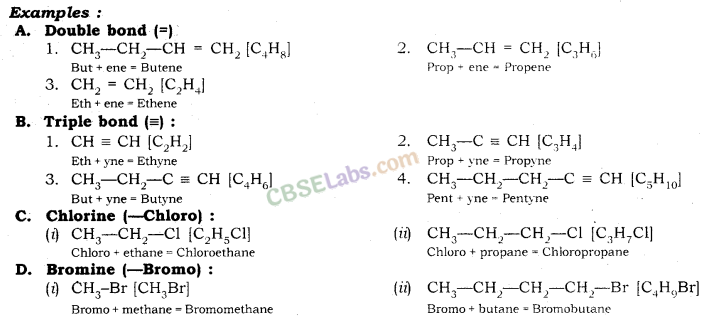
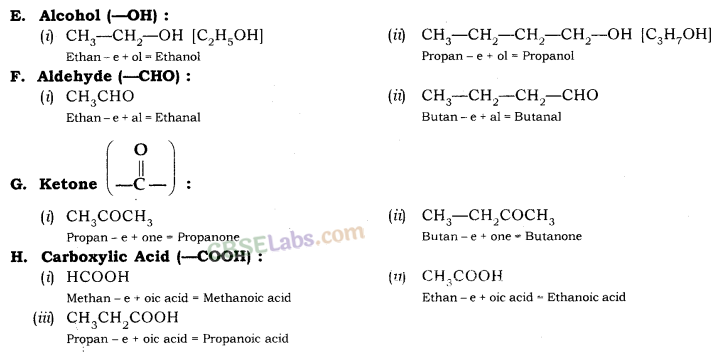
Chemical Properties of Carbon Compounds: The important chemical properties are as follows:
1. Combustion: The complete combustion of carbon compounds in the air gives carbon dioxide water, heat and light.
CH3CH2OH(l) + O2(g) → CO2(g) + H2O(l) + Heat and light
Carbon burns in air or oxygen to give carbon dioxide and heat and light.
C(s) + O2(g) → CO2(g) + Heat and light
Saturated hydrocarbons burn with a blue flame in the presence of a sufficient supply of air or oxygen.
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) + Heat and light
In presence of limited supply of air, saturated hydrocarbon forms a sooty flame.
Unsaturated hydrocarbons burn with a yellow smoky flame.
The gas and kerosene stove used at home has inlet for air so that, burnt to given clean blue flame.
Due to presence of small amount of nitrogen and sulphur, coal and petroleum produces carbon dioxide with oxides of nitrogen and sulphur which are major pollutant.
2. Oxidation: Oxidation of ethanol in presence of oxidizing agents gives ethanoic acid.
Oxidizing Agent: Some substances are capable of adding oxygen to others, are known as Oxidising Agent.
Example: Alkaline KMnO4 (or KMnO4—KOH)
Acidified K2Cr2O7 (or K2Cr2O7—H2SO4)
KMnO4 – Potassium permanganate
K2Cr2O7 – Potassium dichromate
3. Addition Reaction: Addition of dihydrogen with unsaturated hydrocarbon in the presence of catalysts such as nickel or platinum or palladium are known as Hydrogenation (addition) reaction.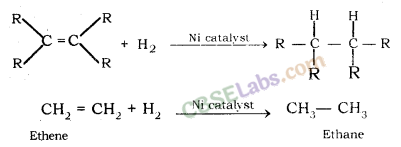
Catalyst: Substances that cause a reaction to occur or proceeds to different rate without consuming in it are called a catalyst. For example; Ni, Pt, Pd, etc.
Process of converting vegetable oil into solid fat (vegetable ghee) is called Hydrogenation of Oil.
Vegetable oil + H2
Vegetable fats are saturated fats which are harmful for health.
Vegetable oil containing unsaturated fatty acids are good for health.
4. Substitution Reaction: Replacement of one or more hydrogen atom of an organic molecule by another atom or group of the atom is known as Substitution Reaction.
Some Important Carbon Compounds :
Ethanol (CH3CH2—OH): Commonly known as Ethyl Alcohol.
Physical Properties
- It is colourless, inflammable liquid.
- It is miscible with water in all proportions.
- It has no effect on the litmus paper.
Chemical Properties
- Reaction with sodium

- Reaction with concentrated H2SO4 (Dehydration Reaction)
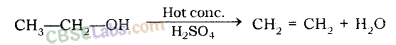
Dehydrating agent: Substances which removes water from ethanol (alcohols) is known as Dehydrating agent. For example; Cone. H2SO4.
Uses: As solvent, as antiseptic (tincture iodine), as anti-freeze in automobiles.
Ethanoic Acid (CH3COOH): Commonly known as Acetic acid. 5-8% of ethanoic acid in water is called Vinegar. The melting point of pure ethanoic acid is 290 K and hence, it often freezes in cold climate so named as glacial acetic acid.
Physical Properties
- It is a colourless, pungent-smelling liquid.
- Miscible with water in all proportions.
- Turns blue litmus to red.
Chemical Properties
(i) Esterification Reaction: Reaction of ethanoic acid with an alcohol in the presence of a few drops of conc. H2SO4 as catalyst gives a sweet-smelling substance known as Esters, called Esterification reaction.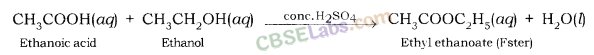
Esters are used in making perfumes and flavouring agents.
Saponification Reaction: Reaction of esters with sodium hydroxide, gives alcohol and sodium salt of carboxylic acid (soap). This reaction is known as Saponification Reaction.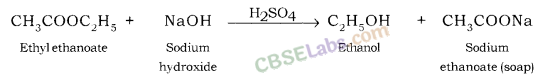
(ii) Reaction with Carbonates and Hydrogen Carbonates: Ethanoic acid reacts with sodium carbonates and sodium hydrogen carbonates to give rise to a salt, carbon dioxide and water.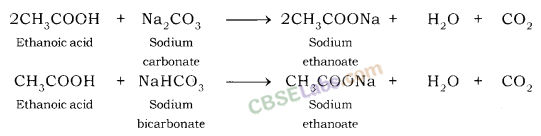
- Used as vinegar.
- Used as raw material for the preparation of acetyl chloride and esters.
Soap: Sodium or potassium salts of long chain fatty acids is called Soap.
General formula: RCOO–Na+
Detergent: Ammonium and sulphonate salts of long chain fatty acids are called Detergent.
Example: CH3—(CH2)11—C6H4—SO3Na.
Hard and Soft Water: Water that does not produce lather with soap readily is called Hard water and which produces lather with soap is called Soft Water.
Hardness of water is due to the presence of bicarbonates, chlorides and sulphate salt of calcium and magnesium.
Difference between soaps and detergents
| Soaps | Detergents |
| (i) These are sodium or potassium salts of long chain fatty acids. | (i) These are ammonium and sulphonate salts of long chain fatty acids. |
| (ii) Ionic part of the soap is —COO–Na+ | (ii) Ionic part of detergent is —OSO3-Na+. |
| (iii) Their efficiency decreases in hard water | (iii) Their efficiency is unaffected in hard water. |
| (iv) Soaps are biodegradable. | (iv) Detergents are non-biodegradable. |
Advantage of Detergents: The main advantage of detergent over soaps is that soaps cannot be used in hard water for washing because hard water reacts with soap to form curdy white precipitate called Scum.
Thus, in hard water, soap does not give lather while detergent does.
Cleansing Action of Soaps and Detergents: Both soaps and detergents cantains two parts. A long hydrocarbon part which is hydrophobic (water repelling) in nature and a short ionic part which is hydrophillic (water attracting) in nature.
The hydrocarbon part of the soap molecule links itself to the oily (dirt) drop and ionic end orients itself towards water and forms a spherical structure called micelles. The soap micelles helps in dissolving the dirt in water and wash our clothes.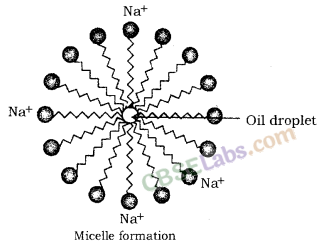
Covalent Bond: A chemical bond formed between two atoms by sharing of valence electrons between two atoms so that each atom acquires the stable electronic configuration of the nearest noble gas.
Covalency: The number of electrons contributed by each atom for sharing.
Carbon always forms a covalent bond: Atomic no of carbon is 6. So, its configuration is K-2, L-4. Therefore, it should either lose or gain 4 electrons to achieve the noble gas configuration and become stable.
However, it is difficult for carbon to gain or lose four electrons because of the following reasons:
- It cannot gain 4 electrons to form C4- ion having Neon gas (2, 8) configuration because this anion would be highly unstable due to a large amount of energy required to overcome the forces of repulsion between the four electrons being added and the six electrons already present in the carbon atom.
- It cannot lose 4 electrons to form C4+ ion having Helium gas (2) configuration because this cation would be highly unstable due to a large amount of energy required to remove four electrons from the carbon atom.
Tetravalency of Carbon: A carbon atom has four electrons in the valence shell. Therefore, carbon forms four covalent bonds, i.e., carbon is tetravalent.
Allotropic forms of Carbon: The phenomenon of existence of an element in two or more forms which have different physical properties but identical chemical properties is called allotropy.
Three allotropic forms of carbon:
- Diamond
- Graphite
- Fullerenes
Hydrocarbon: Organic compounds of carbon and hydrogen are called hydrocarbons.
Saturated Compound: Compounds of carbon which have only single bonds between the carbon atoms are called saturated compounds e.g., Ethane, Propane, Butane etc.
Unsaturated Compound: Compounds of carbon which contain one or more double or triple bonds between carbon atoms are called unsaturated compounds
e.g., Ethene, Propene, Butyne, etc.
Alkanes
- General formula – CnH2n+2
- Saturated hydrocarbons
- Methane – CH4
- Ethane – C2H6
Alkenes
- General formula – CnH2n
- Unsaturated hydrocarbon.
- Ethene – C2H4
- Propene – C3H6
Homologous series: A family of organic compounds having the same functional group, similar chemical properties and the successive (adjacent) members differ by a CH2 unit or 14 mass unit.
Characteristics of a homologous series:
All the members of a homologous series can be represented by a general formula.
- Alkane – CnH2n+2
- Alkyne – CnH2n-2
- Alcohol – CnH2n+1OH
- Ketone – CnH2n+1COCnH2n+1
- Alkene – CnH2n
- Haloalkane – CnH2n+1X
- Aldehyde – CnH2n+1CHO
- Carboxylic acid – CnH2n+1COOH
The molecular formula of two successive (adjacent) members of a homologous series differs by a CH2 unit.
The molecular masses of any two successive members of a homologous series differ by 14 u.
All the members of a given homologous series have the same functional group.
All the members of a series show similar chemical properties.
The members of a homologous series show a gradation in physical properties.
Nomenclature of carbon compound: International Union of Pure and Applied Chemistry (IUPAC) decided some rules to name the carbon compounds. This was done to maintain uniformity throughout the world. Names which are given on this basis are popularly known as IUPAC name. The rules for nomenclature are as follows:
(i) Identify the number of carbon atoms in the carbon compound. Name the carbon compounds according to the number of carbon atoms.
Example, Saturated hydrocarbon having one carbon atom is named as Methane. Saturated hydrocarbon having two carbon atoms is named as Ethane.
- An unsaturated hydrocarbon with a double bond having two carbon atoms is named as Ethene.
- An unsaturated hydrocarbon with a triple bond between carbon atoms is named as Ethyne.
(ii) If the structure has a branched chain, identify the longest chain and then identify the number of carbon atoms.
(iii) In the case of a functional group present, write the prefix or suffix of the functional group as given below. Then write the name of the parent compound:
Chemical properties of Ethanol
- Ethanol (C2H5OH) compound is a colourless liquid at room temperature. It is the second member of the homologous series of alcohols. Its common name is ethyl alcohol. Its functional group is – OH.
- It has a very low melting point (156 K) and low boiling point (351 K or 78°C).
- Ethanol is highly soluble in water.
- Ethanol is one of the main components of alcoholic drinks.
- It is a good organic solvent.
- It is a neutral substance, so it does not have any effect on either blue litmus solution or red litmus solution.
- It bums with a blue flame in the presence of O2 of air. This combustion is an oxidation process.

- In the presence of alkaline KMnO4, it is oxidised to ethanoic acid.
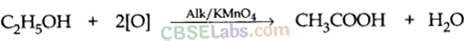
- Ethanol alcohol reacts with sodium(Na) metal vigorously to form sodium ethoxide and evolves H2 gas.
2C2H5OH + 2Na → 2C2H5ONa (Sodium ethoxide) + H2 (g) - Ethanol on dehydration in the presence of cone. H2SO4 acid at 443 K forms ethene gas. H2SO4 acid absorbs water molecules from the alcohol molecules and acts as a strong dehydrating agent.
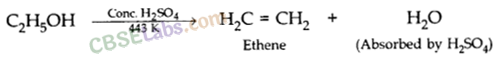
Chemical properties of Ethanoic acid
- Ethanoic acid commonly called acetic acid (CH3COOH) is a colourless liquid. The functional group present in it is carboxylic acid – COOH.
- It’s melting point is 290 K and the boiling point is 391 K.
- Being an acid, it turns blue litmus red.
- It is sour in taste.
- Ethanoic acid reacts with alcohols in the presence of cone. H2SO4 acid to form sweet smelling compounds called esters.
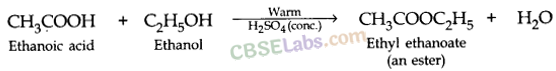
- Ethanoic acid reacts with bases to form its salt and water.
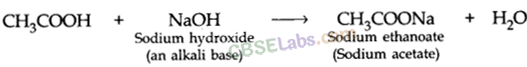
- It reacts with carbonate and hydrogen-carbonate compounds of metals to form its salt (sodium ethanoate commonly called sodium acetate) and release CO2 gas.
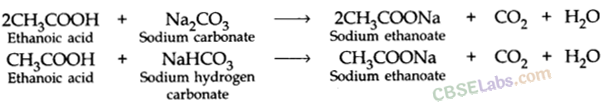
Cleansing action of soap: The dirt is generally held to the surface of a dirty cloth by a thin film of oil or grease.
When a dirty cloth is treated with soap or detergent solution, the non- polar tail of the soap or the detergent dissolve in oil or grease while the polar heads are held by the surrounding water. Soap or detergent micelle is formed with the oily or greasy dirt lying at their Centre (Soap or detergent is attracted both by the greasy dirt and water.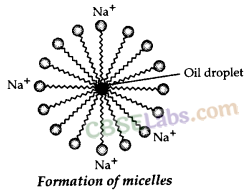
When the surface of the cloth is mechanically scrubbed or beaten on a stone or with a wooden paddle or agitated in a washing machine, the loosened oily particle is removed from the dirty surface and the cloth is cleaned. Detergents lower the surface tension of water to a greater extent than soap, therefore the cleansing action of detergent is much higher than those of soaps.
1. The earth’s crust has only 0.02% carbon in the form of minerals (like carbonates^bicarbonates, coal, and petroleum).
2. The atmosphere has 0.03% of carbon dioxide.
3. In spite of its small amount available in nature, carbon is a versatile element as it forms the basis for all living organisms and many things which we use.
4. Bonding in carbon :
- The atomic number of carbon = 6
- An electronic configuration has 2 electrons in K shell and 4 electrons in L shell.
- In order to attain the noble gas configuration, carbon should either gain 4 electrons or lose 4 electrons or can share it’s 4 electrons with some other element.
- The gain of 4 electrons (to form an octet, i.e., 8 electrons in C4- anion) is difficult because then a nucleus with 6 protons will have to hold extra four electrons.
- Loss of 4 electrons (to attain duplet, i.e., 2 electrons like He atom in C4+ cation) is difficult as it requires a large amount of energy to remove four electrons.
- Carbon, hence, overcomes this difficulty by sharing it’s four valence electrons with other atoms of carbon or with atoms of other elements. These electrons contributed by the atoms for mutual sharing in order to acquire the stable noble gas configuration is called covalency of that atom. Hence, carbon shows TETRACOVALENCY.
- The simplest molecule formed by sharing of electrons (i.e., covalent bonds), can be represented by electron dot structure.
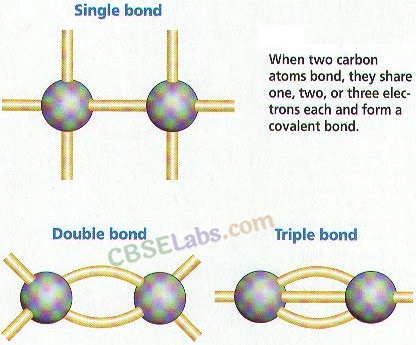
5. Allotropes of carbon: The phenomenon by means of which an element can exist in two or more forms, with similar chemical properties but different physical properties are called allotropy and the different forms are called allotropes. Carbon shows three allotropic forms :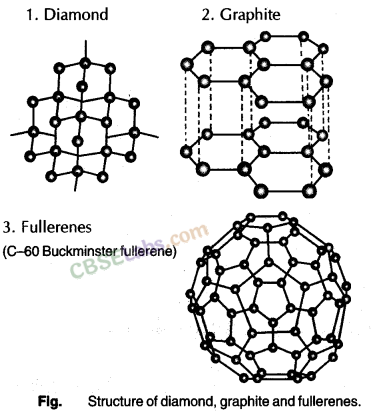
Note:
- Diamond is the hardest substance whereas graphite is very soft.
- Diamond is used for grinding and polishing of ‘ hard materials and graphite is used as a lubricant.
- Diamond has a three-dimensional rigid structure but graphite has a hexagonal sheet layer structure.
- Diamond is a bad conductor of electricity but graphite is a very good conductor of electricity.
6. Fullerenes: A new category of carbon allotrope, fullerenes are spherical in shape or a soccer ball like. The first fullerene identified was C-60 with 60 carbon atoms arranged like the geodesic dome designed by US architect, Buckminster Fuller, hence these are also known as Buckminster Fullerenes or Bucky Ball structures.
7. Cause of versatile nature of carbon: Four main reasons for the versatile nature of carbon are:
(a) Catenation: It is the unique property of self-linkage of carbon atoms by means of covalent bonds to form straight chains, or branched chains, or the rings of different sizes (as shown below):
(b) Tetracovalency: Due to small size, and presence of four valence electrons, carbon can form strong bonds with other carbon atoms, hydrogen, oxygen, nitrogen, or sulphur, etc. For example, compounds of carbon with hydrogen are called hydrocarbons.
(c) Multiple Bond Formation : Small size of carbon also enables it to form multiple bonds, (i.e., double bonds or triple bonds) with other elements as well as with its own atoms. This increases the number of carbon compounds.
Note:
- Compounds of carbon with double bonds and triple bonds are called as unsaturated compounds while those with carbon-carbon single bonds are called saturated compounds.
- Alkenes (with —C = C —) and Alkynes (with —C = C—) are hence unsaturated, whereas Alkanes (with — C — C—) are saturated compounds.
(d) Isomerism: The phenomenon by means of which the carbon compounds with same molecular formula show different structures, and properties, e.g., A chain of 4 carbon atoms can be written in two ways :
Hence, the number of carbon compounds increases to a huge number.
8. Hydrocarbons: a Large number of hydrocarbons can be classified as: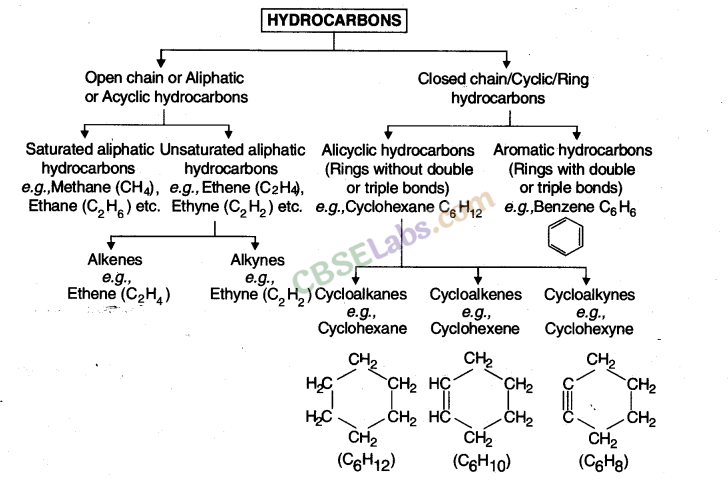
Note: In an open chain, the name of parent chain is derived from the root word and suffix ane, ene or yne is added depending on the type of bond present in a chain :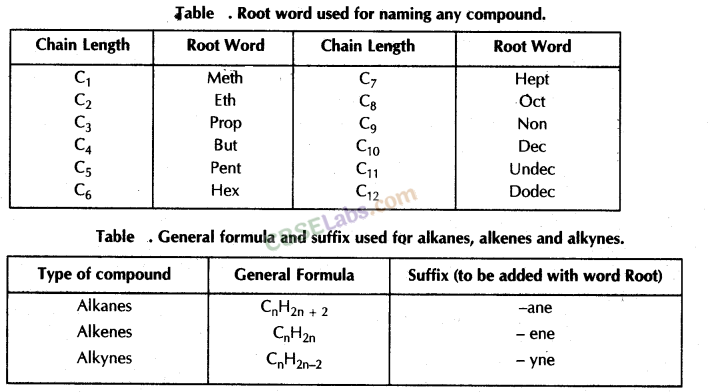
Important: No alkene or alkyne is possible with single carbon atom because double or triple bond is not possible between carbon and hydrogen atom. It is only between two carbon atoms.
9. Functional Group:
- An atom or a group of atoms which when present in a compound gives specific properties to it, is called a functional group.
- A single line shown along with a functional group is called as its free valency by which it gets attached to a compound by replacing one hydrogen atom or atoms, e.g., -Cl.
- Functional group, replacing the hydrogen is also called as heteroatom because it is different from carbon, and can be nitrogen, sulphur, or halogen, etc.
Important: Replacement of hydrogen atom by a functional group is always in such a manner that valency of carbon remains satisfied.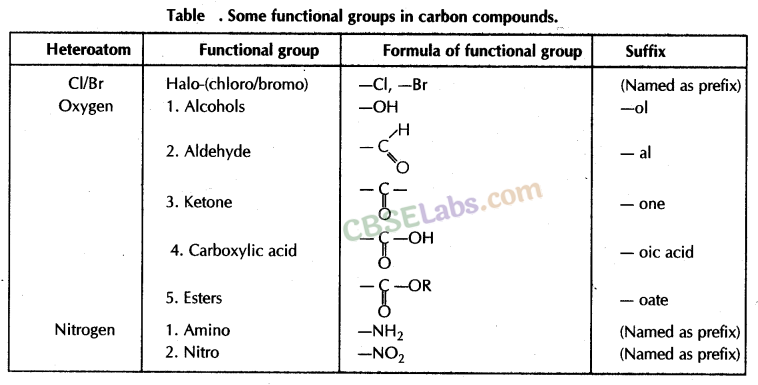
Note : Cl is named as prefix Chloro; Br as Bromo; NH2 as Amino and N02 as Nitro.
Important Note: Symbol ‘R’ in a formula represents an Alkyl Group which is formed by the removal of one hydrogen atom from an alkane.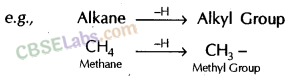
10. Homologous series: A series of organic compounds in which every succeeding member differs from the previous one by -CH2 group or 14 a.m.u.
Note : As the molecular mass increases in a series, : so physical properties of the compounds
show a variation, but chemical properties which are determined solely by a functional group, remains same within a series.
11. Nomenclature of Organic Compounds
- Trivial or common names: These names were given after the source from which the organic compounds were first isolated, e.g., If a compound has one carbon atom, then its common name will have root word form and so on (see table).
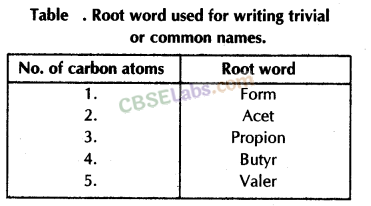
- IUPAC name: International Union of Pure and Applied Chemistry gave following rules for naming various compounds :
- Identify the number of carbon atoms and write the word root corresponding to it. e.g., If a number of carbon atoms is three, then the word root is a prop.
- Presence of a functional group is indicated by prefix or suffix as given in table 2, and table 3.
- If the name of the functional group is to be given as a suffix, the last letter ‘e’ in the name of the compound is deleted and the suffix is added. e.g., a ketone with three carbon atoms is named as :
Propane – e = Propan + ‘one’ = Propanone. Alcohol with three carbons is propanol. Carboxylic acid with three carbons is propanoic acid. - Halogens, in IUPAC, are written as Prefixes, e.g., Compound With two carbons and one chloro group is named as chloroethane (CH3CH2CI).
12. Chemical properties of carbon compounds :
Main properties of carbon compounds are :
(a) Combustion Reaction
(b) Oxidation Reaction
(c) Addition Reaction.
(d) Substitution Reaction
(a) Combustion Reaction: A chemical reaction in which a substance burns in the presence of air or oxygen is called combustion reaction.
Note: Combustion is always an EXOTHERMIC reaction, e.g.,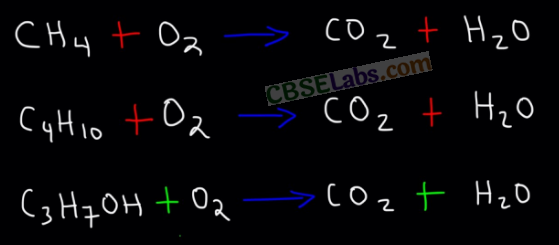
Remember:
- Saturated hydrocarbons generally give clean flame whereas unsaturated hydrocarbons give sooty flame (because carbon content is more than hydrogen content in these, and hence carbon shows incomplete combustion and appears as soot).
- Saturated hydrocarbons can give sooty flame in a limited supply of oxygen.
(b) Oxidation Reaction: The addition of oxygen in a compound upon combustion is called oxidation.
In addition to combustion, oxidation can also be : brought about by some substances which are
capable of giving oxygen to others, i.e., Oxidising agents, e.g., Acidified K2Cr207 (Potassium dichromate) and alkaline KMn04 (Potassium permanganate).
Note: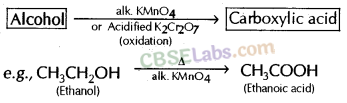
(c) Addition Reaction: Addition of a molecule in unsaturated compounds in the presence of a catalyst, to give saturated compound is called an addition reaction, e.g.,
Hydrogenation of vegetable oils as shown in the reaction below :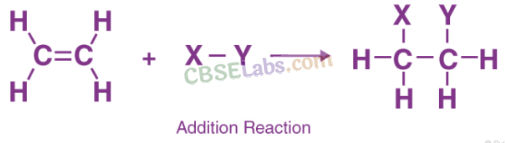
(d) Substitution Reaction: The reactions which involve the replacement of an atom or group of atoms from a molecule by another atom without any change in structure in the remaining part of the molecule.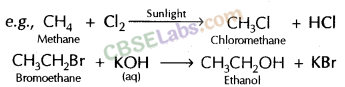
13. Ethanol: (or alcohol)
Colourless liquid, soluble in water, and has a distinct smell and burning taste. Its consumption in small quantities causes drunkenness and can be lethal.
14. Ethanoic Acid: CH3COOH
Common Name: Acetic Acid.
5-8% solution of acetic acid in water is called Vinegar. And 100% pure acetic acid is called Glacial acetic acid because it has m.pt. 290 K and freezes forming glacier like crystals.
Reactions of ethanoic acid :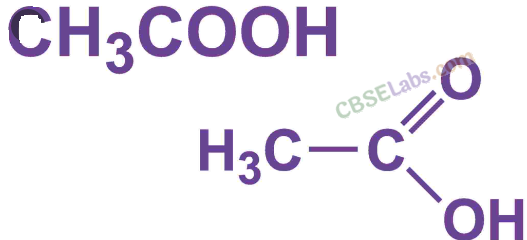
Saponification : Esters in the presence of acid or base react to give back alcohol and carboxylic acid is called saponification.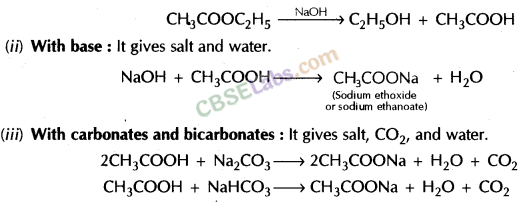
15. Soaps and Detergents :
Soaps and Synthetic Detergents: Soaps and detergents are substances used for cleaning.
Soap: Soaps are sodium or potassium salts of higher fatty acids, such as Oleic acid (C17H33COOH), Stearic acid (C17H35COOH), Palmitic acid (C15H31COOH), etc. These acids are present in the form of their esters along with glycerol (alcohol containing three hydroxyl groups). These esters, called ‘glycerides’ are present in fats and oils of animal and vegetable origin.
Preparation of Soap: When an oil or fat (glyceride) is treated with sodium hydroxide solution, it gets converted to sodium salt of the acid (soap) and glycerol. The reaction is known as saponification.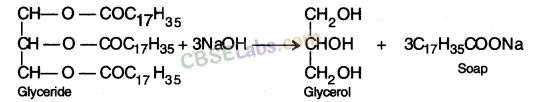
Detergents: Chemically, detergents are sodium salts of sulphonic acids, i.e., detergents contain a sulphonic acid group (—S03H), instead^of a carboxylic acid group (—COOH), on one end of the hydrocarbon.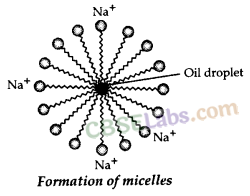
The cleansing action of detergent is considered to be more effective than a soap.
Cleansing Action of Soaps and Detergents: The cleansing action of soaps and detergents follows the same principle.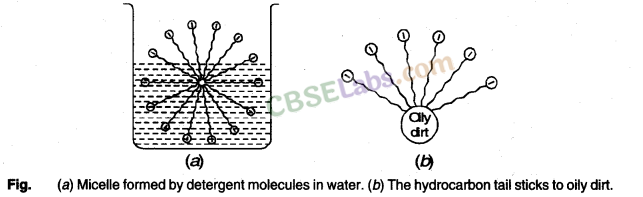
When soap or detergent is dissolved in water, the molecules gather together as clusters, called micelles. The tails stick inwards and the heads outwards.
In cleansing, the hydrocarbon tail attaches.itself to oily dirt. When water is agitated, the oily dirt tends to lift off from the dirty surface and dissociates into fragments. This gives an opportunity to other tails to stick to oil. The solution now contains small globules of oil surrounded by detergent molecules. The negatively charged heads present in water prevent the small globules from coming together and form aggregates. Thus, the oily dirt is removed from the object.
16. Scum: The insoluble precipitates formed by soap molecule when they react with calcium and magnesium ions present in hard water. Due to this, a lot of soap gets wasted and cleansing action gets reduced to a larger extent.
NCERT Exemplar Class 10 Science Chapter 4 Carbon and its Compounds
Short Answer Questions
Question.1 Draw the electron dot structure of ethyne and also draw its structural formula.
Answer.
Question.2 Write the names of the following compounds.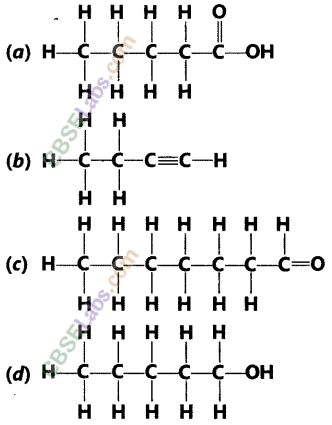
Answer.
(a) Pentanoic acid (b) 1-Butyne
(c) Heptanal (d) 1-Pentanol
Question.3 Identify and name the functional groups present in the following compounds.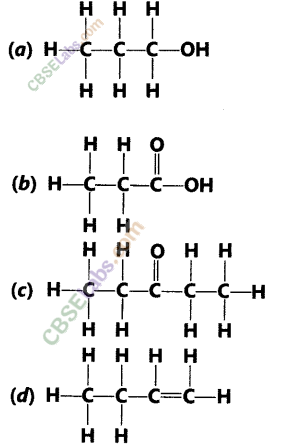
Answer.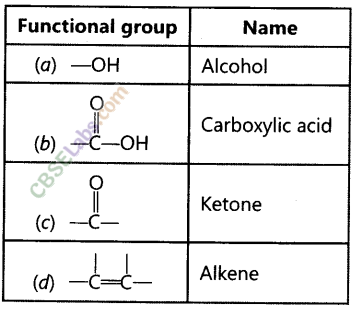
Question.4 A compound X is formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of
(a) carboxylic acid, (6) alcohol and (c) the compound X. Also write the
reaction.
Answer.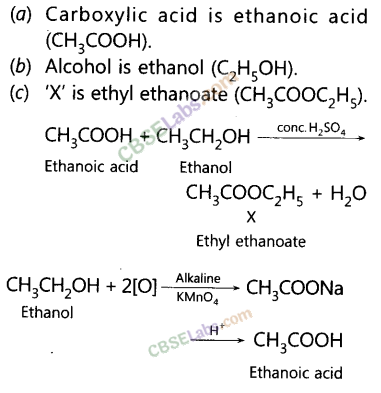
Question.5 Why detergents are better cleansing agents than soaps? Explain.
Answer. It is because detergents form lot of lather even with hard water.
Hard water contains Ca2+ and Mg2+ ions which react with soap to form insoluble salts of calcium and magnesium called scum and soap goes waste. Detergents do not form insoluble compounds with Ca2+ and Mg2+ ions, therefore, these are more effective.
Question.6 Intake of small quantity of methanol can be lethal. Comment.
Answer. Methanol is oxidised to methanal in liver.’ Methanal is highly reactive and good reducing agent. It causes protoplasm to coagulate. It also affects optic nerve and leads to blindness.
Question.7 A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
Answer. Hydrogen gas is evolved.
Question.8 Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
Answer. Carbon shows catenation to large extent as compared to silicon as well as any other element due to smaller size of carbon. C—C bond is stronger than Si-Si bond because Si is larger in size, forms wea’ker bond.
Question.9 Match the reactions given in Column (A) with the names given in Column (B).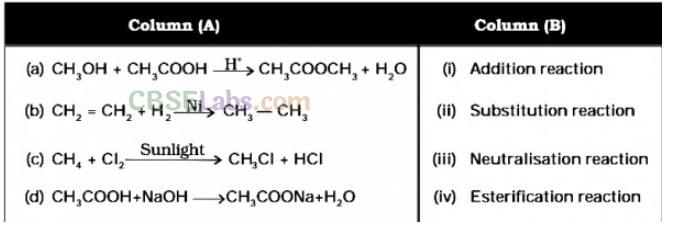
Answer.
(a) – (iv) is esterification reaction because ester is being formed from carboxylic acid and alcohol.
(b) – (i) is addition reaction as hydrogen is being added.
(c) – (ii) is substitution reaction because hydrogen of methane is being substituted by
chlorine atom.
(d) – (iii) is neutralisation reaction because acetic acid reacts with sodium hydroxide to form salt and water.
Question.10 What is the role of metal or reagents written on arrows in the given chemical reactions?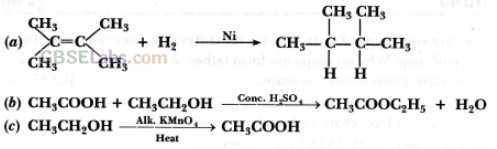
Answer.
(a) Ni is used as a catalyst.
(b) Cone. H2SO4 acts as a dehydrating agent.
(c) Alkaline KMnO4 is an oxidising agent.
Long Answer Questions
Question.11 (a) Write the formula and draw electron dot structure of carbon tetrachloride.
(b) What is saponification? Write the reaction involved in this process.
Answer.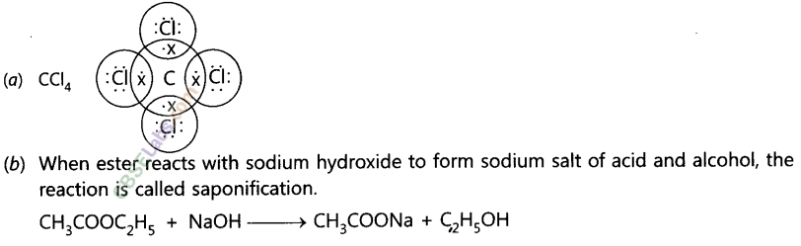
Question.12 A compound ‘C’ (molecular formula, C2H4O2) reacts with Na-metal to form a compound ‘R’ and evolves a gas which burns with a pop sound. Compound ‘C’ on treatment with an alcohol ‘A’ in presence of an acid forms a sweet smelling compound ‘S’ (molecular formulaC3H6O2). On addition of NaOH to ‘C’, it also gives ‘R’ and water. ‘S’ on treatment with NaOH solution gives back ‘R’ and ‘A’.
Identify ‘C’, ‘R’, ‘A’, ‘S’ and write down the reactions involved.
Answer.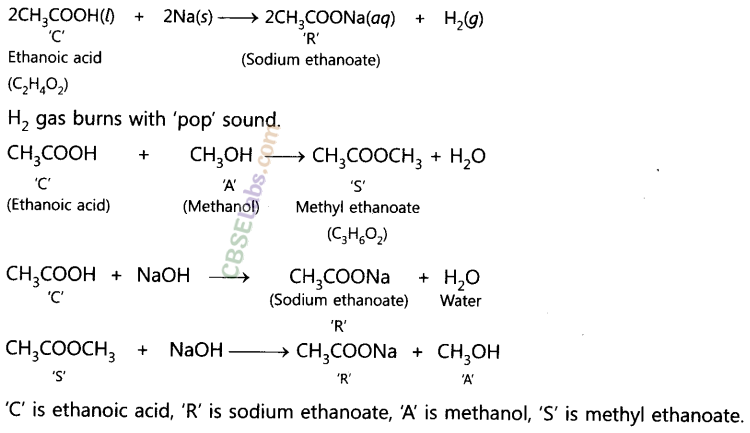
Question.13 How would you bring about the following conversions? Name the process and write the reaction involved.
(a) Ethanol to ethene
(b) Propanol to propanoic acid
Answer.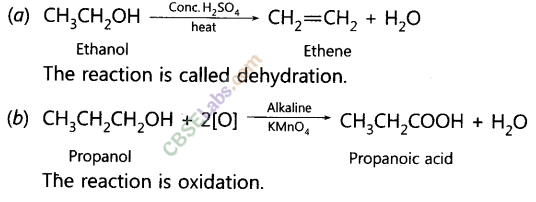
Extra Questions Carbon and its Compounds – CBSE Class 10 Science
According to new CBSE Exam Pattern, MCQ Questions for Class 10 Science pdf Carries 20 Marks.
Question-1
A piece of black electrode used in dry cell on strong heating in air gave a colourless gas which turned lime -water milky. What was the material of the electrode?
Solution:
We know that graphite is used for making the electrodes. So, the piece of black electrode used in the dry cell is made of graphite (which is an allotrope of carbon element). This is confirmed by the fact that the piece of electrode, on strong heating in air, gave a colourless gas carbon dioxide which turned lime- water milky. Thus, the material of the electrode is graphite.
Question-2
Why does graphite conducts electricity, but not diamond?
Solution:
In case of diamond, each carbon atom of a single crystal is surrounded by four other carbon atoms by covalent bonds such that they form four corners of a regular tetrahedron. Because of four covalent bonds with each carbon atoms there are no free electrons available. Due to the non-availability of free electrons within crystalline structure, diamond acts as a bad conductor of electricity.
In case of graphite, every carbon atom in a single crystal is covalently bonded to three carbon atoms. As each carbon atoms has four valence electrons one valence electron is left free for each carbon atom. These free electrons can be easily made to flow within the crystalline structure of graphite by applying electric potential. Thus, graphite is a good conductor of electricity.
Question-3
Write three important uses of ethanol.
Solution:
Ethanol is used as a solvent to dissolve varnishes, medicines and other organic compounds
It is used as beverage (for drinking as an intoxicant) in different forms, viz; Brandy, Whisky etc.,
It is used for industrial uses in the name of denatured spirit.
Question-4
State what you will observe when sugar crystals is heated strongly.
State what you will observe when sugar crystals is treated with conc. Sulphuric acid.
Solution:
The sugar crystal will initially melt. Gradually, they turn brown and start swelling up. They give off large amount of steam. Finally black porous residue of carbon is left behind.
The sugar crystals will initially turn brown. Lot of frothing takes place with the evolution of large amount of heat and steam is given off. Finally a black porous residue of carbon is left behind.
Question-5
How are the molecules of aldehyde and Ketone structurally different?
Solution:
In aldehyde, the carbon atom of the carbonyl group is attached to one alkyl group (R) and one hydrogen atom but in ketone, the carbonyl group is attached to two alkyl groups.
Question-6
What change has been made in the composition of detergents to make them biodegradable?
Solution:
Detergents made from long chain hydrocarbons having the minimum branching in their molecules are degraded more easily.
Question-7
A hydrocarbon molecule contains 4 hydrogen atoms. Give its molecular formula, if it is an: (i) alkane, (ii) alkene (iii) alkyne.
Solution:
(i) An alkane containing 4 hydrogen atoms in its molecule is methane, CH4.
(ii) An alkene containing 4 hydrogen atoms in its molecule is ethane, C2H4
(iii) An alkyne containing 4 hydrogen atoms in its molecule is propyne, C3H4.
Question-8
Why common salt is added in soap making?
Solution:
Common salt is added to the mixture to make the soap come out of solution. Though most of the soap separates out on its own but some of it remains in solution. Common salt is added to precipitate out all the soap from the aqueous solution. Actually, when we add common salt to the solution, then the solubility of soap present in it decreases, due to which all the soap separates out from the solution in the form of a solid.
Question-9
What is meant by denatured alcohol? What is the need to denature alcohol?
Solution:
The alcohol which is rendered unfit by mixing it with some poisonous substances, such as methanol, pyridine, copper sulphate, etc is known as denatured alcohol. Ethanol is an important industrial chemical. Therefore, it subjected to very small excise duty. To prevent its misuse for drinking purpose, there is a need to denature alcohol.
Question-10
What is meant by the term “functional group”?
Solution:
A functional group in an organic compound is an atom or a group of atoms binded together in a unique fashion, which is usually the site of chemical reactivity in an organic molecule.
NCERT Solutions for Class 10 Science All Subject NCERT Solutions
- Chapter 1 Chemical Reactions and Equations
- Chapter 2 Acids, Bases and Salts
- Chapter 3 Metals and Non-metals
- Chapter 4 Carbon and Its Compounds
- Chapter 5 Periodic Classification of Elements
- Chapter 6 Life Processes
- Chapter 7 Control and Coordination
- Chapter 8 How do Organisms Reproduce?
- Chapter 9 Heredity and Evolution
- Chapter 10 Light Reflection and Refraction
- Chapter 11 Human Eye and Colourful World
- Chapter 12 Electricity
- Chapter 13 Magnetic Effects of Electric Current
- Chapter 14 Sources of Energy
- Chapter 15 Our Environment
- Chapter 16 Management of Natural Resources
.png)
.png)